Mechanosensitive biochemical imprinting of the talin interaction with DLC1 regulates RhoA activity and cardiomyocyte remodeling
- PMID: 40911671
- PMCID: PMC12412656
- DOI: 10.1126/sciadv.adt6083
Mechanosensitive biochemical imprinting of the talin interaction with DLC1 regulates RhoA activity and cardiomyocyte remodeling
Abstract
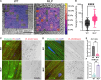
During heart disease, the cardiac extracellular matrix (ECM) undergoes a structural and mechanical transformation. Cardiomyocytes sense the mechanical properties of their environment, leading to phenotypic remodeling. A critical component of the ECM mechanosensing machinery, including the protein talin, is organized at the cardiomyocyte costamere. Our previous work indicated a different talin tension, depending on the ECM stiffness, but the effects on downstream signaling remained elusive. Here, we identify that the talin interacting proteins DLC1 (deleted in liver cancer 1), RIAM (Rap1-interacting adaptor molecule), and paxillin each preferentially bind to talin at a specific ECM stiffness, this interaction is preserved in the absence of tension, and the interaction is regulated through focal adhesion kinase signaling. Moreover, DLC1 regulates cardiomyocyte RhoA activity in a stiffness-dependent way, whereby the loss of DLC1 results in myofibrillar disarray. Together, this study demonstrates a mechanism of imprinting mechanical information into the talin interactome to fine-tune RhoA activity, with impacts on cardiac health and disease.
Figures





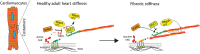
References
-
- Ruotsalainen H., Sipila L., Kerkela E., Pospiech H., Myllyla R., Characterization of cDNAs for mouse lysyl hydroxylase 1, 2 and 3, their phylogenetic analysis and tissue-specific expression in the mouse. Matrix Biol. 18, 325–329 (1999). - PubMed
-
- Lopez B., Gonzalez A., Hermida N., Valencia F., de Teresa E., Diez J., Role of lysyl oxidase in myocardial fibrosis: From basic science to clinical aspects. Am. J. Physiol. Heart Circ. Physiol. 299, H1–H9 (2010). - PubMed
-
- Maki J. M., Lysyl oxidases in mammalian development and certain pathological conditions. Histol. Histopathol. 24, 651–660 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

