Moderating effects of plasma glial fibrillary acidic protein along the Alzheimer's disease continuum
- PMID: 40911721
- PMCID: PMC12412752
- DOI: 10.1002/alz.70626
Moderating effects of plasma glial fibrillary acidic protein along the Alzheimer's disease continuum
Abstract
Introduction: Glial fibrillary acidic protein (GFAP) may contribute to Alzheimer's pathology at early disease stages. GFAP moderation of Alzheimer's disease (AD)-related neurodegeneration and cognition is unclear.
Methods: We examined plasma GFAP moderation of AD biomarkers (amyloid beta [Aβ]-positron emission tomography [PET][A]; plasma phosphorylated tau-181 [p-tau181][T1]), neurodegeneration (plasma NfL[Nplasma]; structural magnetic resonance imaging [MRI][NMRI]), and cognition (Cogmemory; Cogexecutive) in two cohorts: University of California San Francisco (UCSF) (N = 212, 91.0% non-Hispanic/Latino White [NHLW], age = 74.7 [7.6] years, 75.9% cognitively unimpaired [CU]) and 1Florida Alzheimer's Disease Research Centers (1FLADRC; N = 582, 32.8% NHLW, age = 70.7 [8.5] years, 28.9% CU).
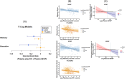
Results: Plasma GFAP consistently moderated A-T1 (UCSF: β = 0.46, p = 0.012; 1FLADRC: β = 0.12, p = 0.029). The association between elevated Aβ-PET and increased (p-tau) was strengthened at higher GFAP concentrations. In 1FLADRC, GFAP moderated T1-Nplasma/MRI. In UCSF, GFAP moderated T1-Cogmemory/executive and NMRI-Cogmemory/executive. Higher GFAP consistently related to worse neurodegeneration and cognition (main effects).
Discussion: Across demographically and clinically heterogeneous cohorts, plasma GFAP is a key moderator of AD and may help identify individuals at greatest risk of AD-related neurodegeneration and cognitive decline.
Highlights: AD biomarkers were measured in two demographically and clinically distinct cohorts. Plasma GFAP moderated Aβ-PET to p-tau associations in both UCSF and 1FLADRC. Cohort-dependent, GFAP moderated p-tau to neurodegeneration and cognition associations. All moderations revealed strengthened disease associations with higher plasma GFAP. Plasma GFAP may help identify individuals at greatest risk of AD-related decline.
Keywords: ATN; Alzheimer's disease; GFAP; astrocyte reactivity; cognition; glial fibrillary acidic protein; inflammation; neurodegeneration; neuroinflammation; neuropathology; plasma GFAP; plasma biomarkers.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Authors report no disclosures relevant to the content of this study. Dr. Armstrong reported grants from the National Institutes of Health (NIH), Florida Department of Health, and Lewy Body Dementia Association Research Center of Excellence; speaker honoraria from the Taiwan International Congress of Parkinson's Disease and Movement Disorders, American Academy of Neurology Annual Meetings, World Congress on Parkinson's Disease and Related Disorders, PRIME CME Program, Dr. Daniel I. Kaufer Lecture Series at the University of Wisconsin Alzheimer's Disease Research Center, Dementia with Lewy Bodies: Filling the Gaps in Translational and Clinical Research NIA‐NINDS Conference, and Michael J. Fox Foundation Neuro‐Impact Workshop; personal compensation for serving as a Data Safety Monitoring Board member with the Alzheimer's Therapeutic Research Institute/ Alzheimer's Clinical Trials Consortium, Alzheimer's Disease Cooperative Study, and an NIH study (R01AG083828); and a non‐compensated relationship as a member of the Scientific Advisory Board Executive Committee for the Lewy Body Dementia Association. Dr. Casaletto reported grants from the National Institute on Aging (NIA), Hillblom Foundation, Alzheimer's Association, and Wellcome Trust, Leap; and non‐compensated relationships as Chair of the International Neuropsychological Society Conflict of Interest Committee and Executive Board member of the Alzheimer's Association ISTAART Cognition Professional Interest Area. Dr. DeKosky reported royalties from UpToDate as the section editor for dementia; consulting fees from Brainstorm Cell Therapeutics, Eisai, and Sanofi; personal fees from Acumen Pharmaceuticals, Biogen, Prevail Pharmaceuticals, Cognition Therapeutics, Vaccinex, Lilly Pharmaceuticals, Nido Biosciences, Neuvivo Pharmaceuticals, Novo Nordisk, and Capricor for serving on the data safety monitoring or medical advisory boards; and honoraria from
Figures



References
MeSH terms
Substances
Grants and funding
- P30AG062422/NIA)
- AARF-23-1145318/Alzheimer's Association Research
- 2024-A-001-CTR/Larry L. Hillblom Foundation
- R01 AG048234/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- R01AG045611/NIA)
- K23 AG058752/AG/NIA NIH HHS/United States
- UF1NS100608/NIA)
- R01 AG072475/AG/NIA NIH HHS/United States
- K23AG084883/NIA)
- 2024-001-1/New Vision Research Charleston Conference on Alzheimer's Disease
- P30 AG066506/AG/NIA NIH HHS/United States
- R01 AG045611/AG/NIA NIH HHS/United States
- AARF-22-974065/Alzheimer's Association Research
- R01AG072475/NIA)
- K23 AG084883/AG/NIA NIH HHS/United States
- K23AG058752/NIA)
- R01AG032289/NIA)
- R01AG048234/NIA)
- R01 AG032289/AG/NIA NIH HHS/United States
- NIH)
- K23AG073514/NIA)
- 2018-A-006-NET/Larry L. Hillblom Foundation
- K23 AG073514/AG/NIA NIH HHS/United States
- AARG-20-683875/Alzheimer's Association Research
- UF1 NS100608/NS/NINDS NIH HHS/United States
- P30AG066506/NIA)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

