Pyrimidine Nucleos(t)ide Therapy in Patients With Thymidine Kinase 2 Deficiency: A Multicenter Retrospective Chart Review Study
- PMID: 40911819
- PMCID: PMC12413740
- DOI: 10.1212/WNL.0000000000213908
Pyrimidine Nucleos(t)ide Therapy in Patients With Thymidine Kinase 2 Deficiency: A Multicenter Retrospective Chart Review Study
Abstract
Background and objectives: Thymidine kinase 2 deficiency (TK2d) is an ultra-rare, progressive, and life-threatening mitochondrial myopathy caused by pathogenic variants of the thymidine kinase 2 gene. Patients often lose the ability to walk, eat, and breathe independently. There are no approved therapies; however, preclinical studies of pyrimidine nucleos(t)ide therapy have shown promising results. We investigated the safety and efficacy of pyrimidine nucleos(t)ide therapy in patients with TK2d.
Methods: Medical records of children and adults with TK2d receiving pyrimidine nucleosides or nucleotides (≤800 mg/kg/d deoxycytidine and deoxythymidine [or their monophosphates]) were collected retrospectively from clinical sites globally. Data included baseline characteristics, medical history, disease-related outcomes, and treatment safety. To assess survival benefit, treated patients were compared with untreated TK2d controls from the literature.
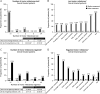
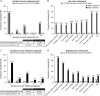
Results: Baseline demographics and clinical characteristics were comparable between treated (n = 38) and untreated (n = 69) patients. Among treated patients (55.3% male; 44.7% female), the median age of TK2d symptom onset was 2.46 years. None of the treated patients (0/38) and 58% (40/69) of untreated patients died. For time to death from TK2d symptom onset or treatment start, treated patients had a reduced risk of death compared with untreated patients (85%-93% [hazard ratio 0.067-0.147] vs 75%-91% [0.091-0.251], respectively). Exact conditional logistic regression analyses confirmed a 95% reduction in risk of death (odds ratios 0.044-0.047; all p < 0.0001). Before treatment, 71.1% (27/38) of patients lost ≥1 motor milestone and 3.7% (1/27) regained a milestone; during treatment, no patients lost milestones and 65.4% (17/26) regained ≥1. Similarly, 28.6% (6/21) of treated patients showed decreased ventilatory support duration, and none (0/21) showed increased duration. Of 8 patients on feeding support when starting treatment, 3 discontinued support. Most treatment-emergent adverse events (TEAEs) were mild (63.2%) and did not lead to discontinuation. No serious TEAEs experienced by more than 1 patient were treatment-related. Findings among patients with TK2d symptom onset of ≤12 years (n = 29) were similar to those of the overall group.
Discussion: These results indicate that pyrimidine nucleos(t)ide therapy was generally well tolerated; had an acceptable safety profile; and may reduce risk of death, positively change disease trajectory, and stabilize or improve symptoms in patients with TK2d.
Trial registration information: ClinicalTrials.gov: NCT03701568. First submitted: September 27, 2018; first participant consented: October 30, 2018. clinicaltrials.gov/study/NCT03701568.
Classification of evidence: This study provides Class III evidence for a reduction in the risk of death in patients with TK2d treated with pyrimidine nucleos(t)ides.
Conflict of interest statement
C. Domínguez-González has received speaker honoraria and has served as scientific advisor for UCB. C. Chiang, A.-O. Colson, I. Rebollo Mesa, and S. VanMeter are employees and stockholders of UCB. E. Baixauli is an employee of Oxford PharmaGenesis. J. Quan was previously employed by Modis Therapeutics/Zogenix and has equity from employment. M. Hirano serves on the advisory board of UCB; has received research support, honoraria, or both from Stealth BioTherapeutics, Entrada Therapeutics, Precision BioSciences, and Modis Therapeutics (a wholly owned subsidiary of Zogenix/UCB); and has received grant support from the NIH (U54 NS078059 and P01 HD32062), Department of Defense (FPA W81XWH2010807), Muscular Dystrophy Association (577392), and J. Willard and Alice S. Marriott Foundation. Columbia University Irving Medical Center (CUIMC) has the patent for deoxynucleos(t)ide therapies for mitochondrial DNA depletion syndrome, including TK2 deficiency, which is licensed to Modis Therapeutics, a wholly owned subsidiary of Zogenix/UCB; this relationship is monitored by an unconflicted external academic researcher. M. Hirano is a coinventor of this patent. CUIMC has received royalty payments related to the development and commercialization of the technology. M. Hirano has received shares of the royalty payments following Columbia University policies; and is on the scientific and medical advisory boards of the United Mitochondrial Disease Foundation and Barth Syndrome Foundation; and is on the Research Advisory Committee of the Muscular Dystrophy Association. Go to
Figures

Comment in
-
Nucleos(t)ide Therapy for TK2-Deficient Mitochondrial Myopathy: Small Molecules With Big Potential.Neurology. 2025 Sep 23;105(6):e214187. doi: 10.1212/WNL.0000000000214187. Epub 2025 Sep 5. Neurology. 2025. PMID: 40911820 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
