A new gut pathogenic bacteria and its metabolites promote colorectal cancer development and act as non-invasive early diagnostic biomarkers
- PMID: 40911847
- PMCID: PMC12416176
- DOI: 10.1080/19490976.2025.2555446
A new gut pathogenic bacteria and its metabolites promote colorectal cancer development and act as non-invasive early diagnostic biomarkers
Abstract
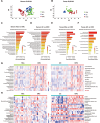
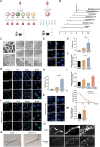
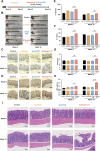
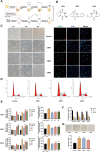
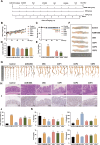
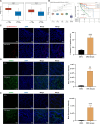
Gut microbiota dysbiosis is strongly linked to colorectal cancer (CRC), but reliable early diagnostic markers remain elusive. This study investigates the role of a novel Shigella flexneri strain in CRC pathogenesis. Metabolomic analysis of CRC patient feces identified elevated agmatine levels. A unique agmatine-producing strain (S. flexneri C.11) was isolated and validated in cell models and pseudo-sterile mice. Affinity fishing combined with HPLC-QTOF-MS characterized bacterial metabolites, while RNA sequencing elucidated mechanistic pathways. Repeated S. flexneri C.11 exposure-induced DNA damage and inflammatory-to-neoplastic transformation. Three genotoxic cyclodipeptides (CDP1-3) were identified, driving malignant transformation and accelerating colitis-associated tumorigenesis. Mechanistically, S. flexneri C.11 upregulated ERBB3, activating the PI3K-AKT pathway. Clinically, combined detection of S. flexneri C.11 and its metabolites differentiated CRC patients from healthy controls (AUC = 0.887), suggesting its potential as noninvasive diagnostic biomarkers for CRC. We identify S. flexneri C.11 as a pro-carcinogenic pathogen and propose ERBB3/PI3K - AKT signaling as a therapeutic target.
Keywords: Colorectal cancer; DNA damage; diagnostic marker; inflammatory bowel disease; intestinal bacteria; metabolite.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early–onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol. 2022;7(3):262–274. doi: 10.1016/S2468-1253(21)00426-X. - DOI - PubMed
-
- Forsberg A, Westerberg M, Metcalfe C, Steele R, Blom J, Engstrand L, Fritzell K, Hellström M, Levin LÅ, Löwbeer C, et al. Screesco investigators. Once–only colonoscopy or two rounds of faecal immunochemical testing 2 years apart for colorectal cancer screening (SCREESCO): preliminary report of a randomised controlled trial. Lancet Gastroenterol. 2022;7(6):513–521. doi: 10.1016/S2468-1253(21)00473-8. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
