Molecular landscape and clinical correlates of olfactory groove meningiomas: a multi-institutional study
- PMID: 40911921
- PMCID: PMC12558015
- DOI: 10.3171/2025.4.JNS242619
Molecular landscape and clinical correlates of olfactory groove meningiomas: a multi-institutional study
Abstract
Objective: The aim of this study was to investigate the relationship between the clinical and radiological characteristics of olfactory groove meningiomas (OGMs) and their molecular profiles.
Methods: The authors performed targeted next-generation and whole-genome sequencing in 123 OGM samples collected from 4 international institutions, focusing on known meningioma-driver genes. They compared the molecular data with the clinical and radiographic features of the tumors. Patient and tumor data, including age, sex, radiological features, and overall survival, were retrospectively collected and analyzed.
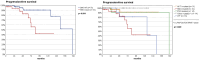
Results: The study cohort comprised 90 females (73%) and 33 males (27%), with a median age at diagnosis of 57 years (range 25-87 years). The majority of tumors (88.6%, n = 109) were classified as WHO grade I meningioma. Known driver mutations were found in 86.2% of patients (n = 106), with the most common mutations found in the SMOL412F/W535L and AKT1E17K genes, each present in 36 cases (29.3%), followed by mutations in PIK3CA/PIK3R1 (19 cases, 15.4%; 14 PIK3CA and 5 PIK3R1), TRAF7 alone (7 cases, 5.7%), POLR2AQ403K (4 cases, 3.3%), and TRAF7/KLF4K409Q (3 cases, 2.4%), while 17 patients (13.8%) did not harbor known meningioma driver mutations (wildtype group). Within molecular subgroups, patients with AKT1 mutations were the youngest (median age 51 years, range 30-87 years) and patients with TRAF7-only mutations were the oldest (median 66 years, range 28-76 years). The median tumor volume at diagnosis was 18.04 cm3. SMO-mutant tumors were significantly larger (median volume 19.5 cm3) than both AKT1-mutant (median 7.5 cm3, p = 0.021) and TRAF7/KLF4-mutant (median 4.9 cm3, p = 0.002) tumors. Tumor-associated hyperostosis of the sphenoid planum was common (58.5%), led by PIK3CA/PIK3R1, SMO, and wildtype groups (73.7%, 72.2%, and 70.6%, respectively), compared with a notably lower rate in AKT1-mutant tumors (25%) (p < 0.001). Tumor invasion of the ethmoid sinuses occurred most frequently in the TRAF7-only mutant OGMs (42.9%), followed by PIK3CA/PIK3R1-mutant (31.6%) and wildtype (23.5%) OGMs. The mean progression-free survival (PFS) was 144.4 months (95% CI 123.8-165 months). Patients with SMO-mutant OGMs exhibited a significantly shorter mean PFS of 92.0 months (95% CI 70.1-113.9 months) compared with 158.2 months (95% CI 134.9-181.5 months) for SMO-wildtype OGMs (p = 0.004), identifying a tumor type that might benefit from adjuvant treatment after resection.
Conclusions: This study revealed that 70% of OGMs harbor SMO, AKT1, and PIK3CA mutations, influencing tumor behavior, symptoms, and outcomes, supporting molecular profiling for personalized treatment in OGM management.
Keywords: AKT1; KLF4; PIK3CA; POLR2A; SMO; TRAF7; molecular; olfactory groove meningioma; oncology; tumor.
Conflict of interest statement
Dr. Cahill reported personal fees from Servier, Boston Scientific, and Incephalo; and advisory board (equity option) from Pyramid Biosciences outside the submitted work. Dr. Brastianos reported consulting for Advise Connect Inspire, Atavistik Bio, and Axiom HealthCare Strategies; personal fees from CraniUS, Genentech, Eli Lilly, InCephalo, Kazia, and MPM; scientific advisory board of CraniUS and Kazia; grant support to MGH and support for clinical trial (including drug) from Mirati Merck and Eli Lilly; clinical trial support/drug for clinical trial grant support from Bristol Myers Squibb, AstraZeneca, Pfizer, and Kazia; grant support to MGH from Kinnate; nonfinancial support from Genentech-Roche (clinical trial support/drug for clinical trial); and non-financial support from Verastem (drug support for research) outside the submitted work. Dr. T. A. Juratli reported personal fees from CSL Behring outside the submitted work.
Figures


References
-
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2016 World Health Organisation Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. - PubMed
-
- Nakamura M, Struck M, Roser F, et al. Olfactory groove meningiomas: clinical outcome and recurrence rates after tumor removal through the frontolateral and bifrontal approach. Neurosurgery. 2007;60(5):844–852. - PubMed
-
- Obeid F, Al-Mefty O. Recurrence of olfactory groove meningiomas. Neurosurgery. 2003;53(3):534–543. - PubMed
-
- Solero CL, Giombini S, Morello G. Suprasellar and olfactory meningiomas. Report on a series of 153 personal cases. Acta Neurochir (Wien) 1983;67(3-4):181–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

