Cholinergic dysfunction in occupational manganese exposure
- PMID: 40912474
- PMCID: PMC12468579
- DOI: 10.1016/j.neuro.2025.103313
Cholinergic dysfunction in occupational manganese exposure
Abstract
Background and objective: Excessive exposure to manganese (Mn) produces a clinical syndrome of parkinsonism and cognitive impairment. However, our understanding of the mechanisms of Mn neurotoxicity remains limited. This study aimed to evaluate the relationships between Mn exposure, cholinergic function, and cognitive impairment in exposed workers.
Methods: We assessed brain cholinergic function using vesicular acetylcholine transporter (VAChT) radiotracer (-)-(1-(8-(2-[(18)F]fluoroethoxy)-3-hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)-piperidin-4-yl)(4-fluorophenyl)methanone (VAT) with positron emission tomography (PET) in 21 Mn-exposed workers. We estimated occupational Mn exposure from work histories and the MRI pallidal index. A cognitive control battery consisting of the Verbal Fluency (VF), Letter Number Sequencing (LNS), Two-Back Letter Task (2B), Go-No-Go (GnG), and Simon Task assessed cognitive function. We applied generalized linear models to Mn exposure, voxel-based cholinergic PET, and cognitive control measures, estimating coefficients for cholinergic-mediated associations between Mn and cognitive function. We utilized bootstrapping techniques to validate the mediation coefficients.
Results: Both Mn exposure metrics were associated with low cholinergic VAT binding in the caudate and cortical regions including the precuneus, pars triangularis, pars opercularis, middle temporal lobe, and entorhinal cortex. Regional cholinergic function mediated the relationship between Mn exposure and both the composite cognitive control score (mean of the 5 cognitive tests) [β = -0.661, 90 % confidence interval (CI) -2.130, -0.032] and the individual VF assessment (β = -0.944, 90 % CI -2.157, -0.065).
Discussion: Higher Mn exposure is associated with lower cholinergic activity in multiple brain regions. Cholinergic function also mediates a portion of the relationship between Mn exposure and cognitive control performance. Caudate and cortical cholinergic activity may be a biomarker of early Mn neurotoxicity and represent an important mechanism of cognitive dysfunction in parkinsonian syndromes.
Keywords: Biomarkers; Cholinergic; Manganese; Neurotoxicology; PET.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest T. Noah Hutson reports no disclosures relevant to the manuscript; S. Searles Nielsen receives support from the following government and non-governmental organizations: R01ES029524NIEHS R01ES029524, R01ES026891, R01ES026891-S1, R01ES025991, R01ES025991-S1, K01ES028295, the US Department of Defense PD190057, and MJFF 000939, 020718; N. Senini reports no disclosures relevant to the manuscript; J. O'Donnell reports no disclosures relevant to the manuscript; H.P. Flores reports no disclosures relevant to the manuscript; T. Hershey receives support from the following government organizations: NIEHS R01ES021488, R01ES029524, R01ES013743; J. S. Perlmutter receives support from the following government and non-governmental organizations: NIA/NINDS RF1NS075321, the American Parkinson Disease Association (APDA) Advanced Research Center at Washington University, the Missouri Chapter of the APDA, the Riney Fund, and the Barnes-Jewish Hospital Foundation; A. K. Soda reports no disclosures relevant to the manuscript; S. M. Moerlein reports no disclosures relevant to the manuscript; Z. Tu receives support from the following government organizations: NIA/NINDS R01NS075527; M. Kasper reports no disclosures relevant to the manuscript; L. Sheppard receives research support from the following government organizations: R01ES029524; B.A. Racette receives research support from the following government and non-governmental organizations: MJFF 000939, 020718, NIEHS R01ES025991, R01ES025991–02S1, R01ES030937-S1,R01ES029524, NIOSH R01OH011661, Department of Defense PD190057, Hope Center for Neurologic Disorders (Washington University), and the Kemper and Ethel Marley Foundation. B.A. Racette has received honoraria (personal compensation) for service on the National Advisory Environmental Health Sciences Council for NIEHS; S.R. Criswell receives research support from the following government and nongovernmental organizations: NIEHS R01ES029524, R01OH011661, R01ES021488.
Figures
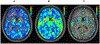


References
-
- Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P, Gauthier S, Kostikov A, Gravel P, Bedard MA, 2017. Quantification of brain cholinergic denervation in alzheimer’s disease using PET imaging with [(18)F]-FEOBV. Mol. Psychiatry 22, 1531–1538. - PubMed
-
- Akamatsu G, Ikari Y, Ohnishi A, Matsumoto K, Nishida H, Yamamoto Y, Senda M, Japanese Alzheimer’s Disease Neuroimaging, I, 2019. Voxel-based statistical analysis and quantification of amyloid PET in the Japanese alzheimer’s disease neuroimaging initiative (J-ADNI) multi-center study. EJNMMI Res. 9, 91. - PMC - PubMed
-
- American Conference Of Governmental Industrial, H. 2013. Annual Reports for the Year 2012: Committees on Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). Cincinnati, OH: ACGIH.
MeSH terms
Substances
Grants and funding
- K24 ES017765/ES/NIEHS NIH HHS/United States
- R01 ES025991/ES/NIEHS NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- R01 NS075527/NS/NINDS NIH HHS/United States
- R01 ES029524/ES/NIEHS NIH HHS/United States
- R01 ES026891/ES/NIEHS NIH HHS/United States
- RF1 NS075321/NS/NINDS NIH HHS/United States
- R01 OH011661/OH/NIOSH CDC HHS/United States
- K23 ES021444/ES/NIEHS NIH HHS/United States
- P42 ES004696/ES/NIEHS NIH HHS/United States
- R01 ES021488/ES/NIEHS NIH HHS/United States
- R01 ES013743/ES/NIEHS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- K01 ES028295/ES/NIEHS NIH HHS/United States
- R01 ES030937/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

