Differential phagocytosis induces diverse macrophage activation states in malignant gliomas
- PMID: 40912739
- PMCID: PMC12414246
- DOI: 10.1136/jitc-2025-012211
Differential phagocytosis induces diverse macrophage activation states in malignant gliomas
Abstract
Background: Diffuse midline glioma (DMG) and glioblastoma (GBM) are aggressive brain tumors with limited treatment options. Macrophage phagocytosis is a complex, tightly regulated process governed by competing pro-phagocytic and anti-phagocytic signals. CD47-SIRPα signaling inhibits macrophage activity, while radiotherapy (RT) can enhance tumor immunogenicity. How RT and CD47 blockade together modulate macrophage "appetite" and activation states remains poorly understood, particularly in the context of glioma immune evasion and therapy resistance.
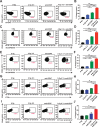
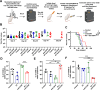
Methods: Human and mouse glioma cell lines were exposed to fractionated RT, anti-CD47 monoclonal antibody, or both. Flow cytometry and ELISA quantified the induction of immunogenic cell death (ICD) and expression of damage-associated molecular patterns (DAMPs). In vitro, phagocytosis assays were performed using peripheral blood mononuclear cell-derived and bone marrow-derived macrophages. Single-cell RNA sequencing (scRNA-seq) was used to analyze transcriptional changes in macrophage subsets that phagocytosed ("eaters") or did not phagocytose ("non-eaters") glioma cells. In vivo, efficacy of combination therapy was assessed using orthotopic xenograft and syngeneic mouse models of DMG and GBM.
Results: RT induced ICD in glioma cells, evidenced by dose-dependent increases in DAMPs such as phosphatidylserine, calreticulin, HSP70/90, and HMGB1. RT and anti-CD47 each promoted macrophage-mediated phagocytosis, with a synergistic effect observed when combined. scRNA-seq of phagocytic macrophages revealed transcriptionally distinct subpopulations associated with each treatment, characterized by enrichment in inflammatory, metabolic, and antigen presentation pathways. In vivo, combination therapy significantly reduced tumor burden, extended survival, and polarized tumor-associated macrophages toward a pro-inflammatory (M1-like) phenotype. Distinct macrophage markers (CLEC7A, CD44, CD63) validated scRNA-seq findings in vivo.
Conclusions: This study highlights that macrophage fate is intimately linked to the molecular properties of what they phagocytose. Phagocytosis is not a singular, uniform process but a dynamic and context-dependent event that drives macrophage specialization and plasticity. By demonstrating that RT and anti-CD47 therapy shape distinct macrophage phenotypes through their effects on tumor immunogenicity, this study provides a framework for understanding how to harness and reprogram macrophage activity for therapeutic benefit. These findings underscore the potential of targeting macrophage plasticity as a strategy to enhance antitumor immunity and improve outcomes in malignant gliomas and other diseases.
Keywords: Combination therapy; Immunotherapy; Innate; Macrophage.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures





Update of
-
Differential Phagocytosis induces Diverse Macrophage Activation States in Malignant Gliomas.bioRxiv [Preprint]. 2025 Mar 17:2025.03.15.642920. doi: 10.1101/2025.03.15.642920. bioRxiv. 2025. Update in: J Immunother Cancer. 2025 Sep 5;13(9):e012211. doi: 10.1136/jitc-2025-012211. PMID: 40166298 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
