Outcomes of patients treated with venetoclax plus azacitidine versus azacitidine alone stratified by advanced age and acute myeloid leukemia composite model
- PMID: 40913104
- PMCID: PMC12589120
- DOI: 10.1038/s41375-025-02730-3
Outcomes of patients treated with venetoclax plus azacitidine versus azacitidine alone stratified by advanced age and acute myeloid leukemia composite model
Abstract
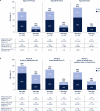
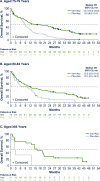
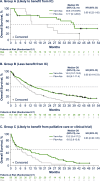
Venetoclax plus azacitidine is recognized as standard of care for patients with acute myeloid leukemia (AML) ineligible for intensive chemotherapy (IC). However, some patients may still not be treated with venetoclax combinations due to frailty concerns. We evaluated efficacy and safety of venetoclax plus azacitidine vs. placebo plus azacitidine in patients with newly diagnosed AML ineligible for IC from the phase 3 VIALE-A study (NCT02993523) and the phase 1b M14-358 study (NCT02203773), stratified by two methods to potentially assess frailty. The first method was age-based (75-79, 80-84, ≥85 years; n = 303 pooled from both studies) and the second was fitness-based using the AML composite model (AML-CM), a comorbidity-based model to estimate mortality risk (Group A, B, C; n = 380, from VIALE-A). Efficacy, including composite complete remission and overall survival, were improved with venetoclax plus azacitidine vs. placebo plus azacitidine across age and AML-CM groups. Safety was generally similar between age and AML-CM groups and no new safety signals were identified. Taken together, these data suggest that patients benefit from venetoclax plus azacitidine regardless of age or degree of frailty and the combination may be considered for patients with AML who may be deemed frail. Clinical trial information NCT02993523; NCT02203773.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: AV: Honoraria from Beigene, AstraZeneca, Pfizer, Amgen, Jazz, AbbVie, Astellas, and Janssen; consulting/advisory role for BMS-Celgene, Istituto Gentili, Kyte-Gilead, Menarinini-Steamline, Glycostem, Servier, Laboratoires Delbert, Novartis, AbbVie, Pfizer, Jazz, and Janssen; speakers’ bureau participation for Pfizer and Servier; travel support from Jazz, Pfizer, AbbVie, Istituto Gentili, and Janssen. J-ZH: Consulting/advisory role for AbbVie, AstraZeneca, and Genentech. PF: Consultancy/advisory role for Bristol Myers Squibb, AbbVie, Janssen, Jazz, and Novartis; research support, as GFM chair, from Bristol Myers Squibb, AbbVie, Janssen, Jazz, and Novartis; honoraria from Bristol Myers Squibb, AbbVie, Janssen, Jazz, and Novartis. BAJ: Consultancy/advisory role for AbbVie, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Gilead, GlycoMimetics, Kymera, Kura, Rigel, Schrodinger, Servier, Syndax, and Treadwell; protocol steering committee for GlycoMimetics; data monitoring committee for Gilead; travel reimbursement from AbbVie and Rigel; research funding to institution from AbbVie, Amgen, Aptose, AROG, Biomea, Bristol Myers Squibb, Celgene, Daiichi Sankyo, F. Hoffmann‑La Roche, Forma, Forty‑Seven, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune‑Onc, Incyte, Jazz, Kymera, Loxo, Pfizer, Pharmacyclics, Sigma Tau, and Treadwell. RV: Honoraria from AbbVie, Astellas, MSD, Novartis, Pfizer, PharmaS, Servier, Teva; speakers’ bureau participation for AbbVie, Astellas, Pfizer, and PharmaS. PM: Consultancy/advisory role for Celgene, Pfizer, and AbbVie; research funding from Pfizer, AbbVie, and Daiichi Sankyo; speakers’ bureau participation for Astellas, Novartis, and Janssen. JSG: Advisory role for AbbVie, Astellas, Bristol Myers Squibb, Genentech, Gilead and Servier. Institutional funding from AbbVie, Genentech, New Wave, Prelude, Pfizer, and AstraZeneca. DR: Speakers’ bureau participation for and honoraria from AbbVie. MJT: Grant support, consulting fees, and advisory board fees from AbbVie, Roche‑Genentech, Janssen, and Pharmacyclics; grant support from Gilead Sciences, Merck, Syndax Pharmaceuticals, TG Therapeutics, and Tolero Pharmaceuticals; and consulting fees and advisory board fees from AstraZeneca and Celgene. MZ, JP, CM, and MD: Employment with AbbVie; may hold stock or other options. VP: Advisory board fees and fees for serving on a speaker’s bureau from AbbVie, Genentech, Pfizer, Jazz, Novartis, Servier, and Amgen.
Figures
References
-
- National Cancer Institute. Cancer stat facts: acute myeloid leukemia. Accessed February 23. (2024). Available at: https://seer.cancer.gov/statistics-network/explorer/.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

