Mitochondrial Complex Member VdNuo1 Recruits Superoxide Dismutases VdSOD2/4 to Maintain Superoxide Anion Homeostasis During Pathogenesis in Verticillium dahliae
- PMID: 40913259
- PMCID: PMC12413312
- DOI: 10.1111/mpp.70149
Mitochondrial Complex Member VdNuo1 Recruits Superoxide Dismutases VdSOD2/4 to Maintain Superoxide Anion Homeostasis During Pathogenesis in Verticillium dahliae
Abstract
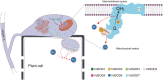
During oxidative phosphorylation, the leaked electrons generate superoxide anions to attack the mitochondrial inner membrane and impair mitochondrial activity. Three superoxide dismutases (SODs) are secreted to degrade host superoxide anions in Verticillium dahliae. However, the roles of mitochondrial SODs (mtSODs) in superoxide anion detoxification and in virulence are unknown in this fungus. We had previously shown that complex I VdNuo1 subunit mediates multiple biological functions and mitochondrial morphogenesis in V. dahliae. Here, we demonstrate that among the seven VdSODs of V. dahliae, only VdSOD2 and VdSOD4 were localised in the mitochondria and interact directly with VdNuo1. The VdNuo1 mutants, which exhibited mitochondrial inactivation in response to the superoxide anion inducer menadione, also displayed aberrant VdSODs transcription and SOD activity. VdSOD2 acted as a positive regulator of mitochondrial superoxide anion detoxification, and thus, its overexpression rescued the menadione-sensitive phenotypes of VdNuo1 mutants. In contrast, the increased tolerance of VdSOD2/VdSOD4 double mutants highlights that VdSOD4 negatively affects superoxide anion degradation. Thus, VdSOD2 and VdSOD4 cooperate with VdNuo1 to maintain SOD homeostasis for growth, mitochondrial superoxide anion detoxification, and virulence in V. dahliae. The two active superoxide anion scavengers CgSOD2 and CgSOD4 shared the same localisation and interaction model with CgNuo1 in Colletotrichum gloeosporioides. These results not only demonstrate the roles of mtSODs in V. dahliae and a novel conserved mechanism in which the respiratory chain couples with mtSODs to regulate superoxide anion metabolism in filamentous fungi, but also provide insights for the development of multisite fungicides to control phytopathogenic pathogens.
Keywords: Verticillium dahliae; mitochondrial superoxide dismutase; pathogenicity; respiratory chain complex I; superoxide anion homeostasis.
© 2025 The Author(s). Molecular Plant Pathology published by British Society for Plant Pathology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
MeSH terms
Substances
Supplementary concepts
Grants and funding
- 2022YFE0111300/National Key Research and Development Program of China
- 2022YFE0130800/National Key Research and Development Program of China
- 32401588/National Natural Science Foundation of China
- 32071768/National Natural Science Foundation of China
- BK2024655/National Natural Science Foundation of Jiangsu Province
LinkOut - more resources
Full Text Sources

