No Increase in Blood Pressure Assessed With the 24-h Holter Monitoring in Patients With Episodic Migraine During Early Treatment With Anti-CGRP Monoclonal Antibodies: A Prospective Observational Study (SAFHYPER)
- PMID: 40913367
- PMCID: PMC12413535
- DOI: 10.1111/ene.70351
No Increase in Blood Pressure Assessed With the 24-h Holter Monitoring in Patients With Episodic Migraine During Early Treatment With Anti-CGRP Monoclonal Antibodies: A Prospective Observational Study (SAFHYPER)
Abstract
Background: Migraine is associated with an increased cardiovascular risk, including hypertension. Anti-calcitonin gene related peptide (CGRP) monoclonal antibodies (mAbs) are effective preventive treatments, but concerns have been raised about their potential hypertensive effects. Herein, we assess the early changes in blood pressure (BP) during anti-CGRP mAbs treatment in patients with migraine using 24-h Holter monitoring.
Methods: We conducted a prospective, real-world study including 20 patients with episodic migraine (EM) during the early treatment with anti-CGRP mAbs. Participants underwent 24-h Holter BP monitoring before treatment and 4 weeks after the first injection. The primary outcome was the change in mean systolic BP (SBP). Secondary outcomes included changes in diastolic BP (DBP), differential BP, diurnal/nocturnal values, heart rate (HR), and dipping patterns.
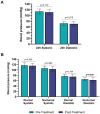
Results: Mean 24-h SBP and DBP showed non-significant differences after treatment (-2.4 mmHg and -1.8 mmHg, p = 0.075). No significant changes were observed in diurnal BP, but a significant reduction in nocturnal DBP was detected (-2.6 mmHg, p = 0.026). Consistently, the proportion of patients with a physiological dipping profile increased from 45.0% to 85.0% post-treatment (p = 0.008). HR remained unchanged, and no patients had mean PAD ≥ 130/80. No adverse events were reported.
Conclusion: Anti-CGRP mAbs did not induce clinically relevant BP increases in the early treatment phase and were associated with improved nocturnal DBP and a favorable shift in dipping profile in patients with EM. These findings suggest the short-term cardiovascular safety of anti-CGRP mAbs, though further studies with larger cohorts and longer follow-up are warranted.
Keywords: CGRP; cardiovascular events; hypertension; migraine; safety.
© 2025 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
L.F.I. received financial support, consulting fees for participation in advisory boards, and support for attending meetings from: Teva, Eli Lilly, Lundbeck, Organon, Pfizer, and AbbVie; he is Associate Editor for Frontiers in Neurology Headache and Neurogenic Pain section. S.G. has received fees and honoraria for advisory boards, speaker panels, or clinical investigation studies from Novartis, Teva, Eli‐Lilly, Pfizer, Lundbeck, Angelini, AbbVie, Orion, and Organon. G.B. has received speaker's fees from Boston, Bayer, Daiichi‐Sankyo, Sanofi, and Janssen outside the submitted work. Other authors have no relevant financial or non‐financial interests to disclose.
Figures

References
-
- Ashina M., “Migraine,” New England Journal of Medicine 383 (2020): 1866–1876. - PubMed
-
- Burch R. C., Buse D. C., and Lipton R. B., “Migraine: Epidemiology, Burden, and Comorbidity,” Neurologic Clinics 37 (2019): 631–649. - PubMed
-
- Wang Y. F. and Wang S. J., “Hypertension and Migraine: Time to Revisit the Evidence,” Current Pain and Headache Reports 25 (2021): 58. - PubMed
-
- Romozzi M., Munafo A., Burgalassi A., et al., “Pharmacological Differences and Switching Among Anti‐CGRP Monoclonal Antibodies: A Narrative Review,” Headache 65 (2025): 342–352. - PubMed
-
- Versijpt J., Paemeleire K., Reuter U., and MaassenVanDenBrink A., “Calcitonin Gene‐Related Peptide‐Targeted Therapy in Migraine: Current Role and Future Perspectives,” Lancet 405 (2025): 1014–1026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

