Performance of non-invasive prenatal testing in vanishing-twin and multiple pregnancies: results of TRIDENT-2 study
- PMID: 40913805
- PMCID: PMC12671934
- DOI: 10.1002/uog.70015
Performance of non-invasive prenatal testing in vanishing-twin and multiple pregnancies: results of TRIDENT-2 study
Abstract
Objective: To evaluate the performance of non-invasive prenatal testing (NIPT) in vanishing-twin and multiple pregnancies.
Methods: This study was conducted as part of the TRIDENT-2 study, in which NIPT was offered as a first-tier screening test to women with a multiple pregnancy or vanishing-twin pregnancy between 1 June 2020 and 31 March 2023 in The Netherlands. Abnormal NIPT results were investigated by follow-up invasive prenatal testing and/or postnatal genetic testing. Chorionicity, amnionicity and type of vanishing twin were determined on first-trimester ultrasound examination. A vanishing twin was classified as Type I in the presence of an additional empty gestational sac or as Type II when an additional non-viable embryo was observed on ultrasound. The performance of NIPT (sensitivity, specificity and positive predictive value (PPV)) was assessed.
Results: NIPT was performed in 655 women with a vanishing-twin pregnancy, of which 231 were Type I, 374 were Type II and 50 were of unknown type. Women with a Type-II vanishing-twin pregnancy had a significantly higher likelihood of receiving an abnormal NIPT result compared to those with Type I (12.6% vs 1.7%; P < 0.001). Among the 655 vanishing twins, NIPT was indicative of trisomies 21, 18 and 13 in 17 cases (screen-positive rate (SPR), 2.60%), four cases (SPR, 0.61%) and eight cases (SPR, 1.22%), respectively. NIPT was indicative of additional findings (chromosomal aberrations other than the major trisomies) in 29 cases that underwent genome-wide NIPT (SPR, 6.16%). In 7/17 (41.2%) cases, trisomy 21 was confirmed in the remaining fetus by cytogenetic follow-up. The sensitivity of NIPT for the detection of trisomy 21 in vanishing-twin pregnancies was 100% (95% CI, 59.0-100%), the specificity was 98.5% (95% CI, 97.2-99.3%) and the PPV was 41.2% (95% CI, 18.4-67.1%). None of the cases of trisomy 18 (n = 4), trisomy 13 (n = 8) or with additional findings (n = 29) were confirmed in the remaining fetus. Of the 12 cases in which NIPT was performed after 15 weeks' gestation, there were no discordant-positive results. NIPT was performed in 2992 women with a dichorionic diamniotic twin pregnancy, 1112 women with a monochorionic twin pregnancy and 75 women with a triplet pregnancy. Of the 2992 dichorionic twin pregnancies, 27 NIPT results were indicative of trisomy 21, 18 or 13 (SPR, 0.90%), of which 21 were confirmed in one fetus. In addition, 16 NIPT results were indicative of an additional finding (SPR, 0.75%), of which three were confirmed by invasive prenatal testing. In 3/1112 (0.3%) monochorionic twin pregnancies, NIPT was indicative of trisomy 21, which was confirmed in both fetuses in all cases. In addition, NIPT was indicative of an additional finding in four cases (SPR, 0.49%), of which none were confirmed. Of the 75 triplet pregnancies, NIPT was indicative of trisomy 21 in one case (SPR, 1.33%); trisomy 21 was confirmed in one of the three triplet fetuses.
Conclusions: Women with a Type-II vanishing-twin pregnancy are more likely to receive an abnormal NIPT result compared to those with a Type-I vanishing-twin pregnancy. NIPT appears suitable for detecting trisomy 21 in the remaining fetus of a vanishing-twin pregnancy, however, none of the trisomy 18, trisomy 13 or additional findings could be confirmed on cytogenetic follow-up. There were no discordant-positive results reported when NIPT was conducted after 15 weeks' gestation. © 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: cell‐free DNA; multiple pregnancy; non‐invasive prenatal testing; prenatal screening; vanishing‐twin pregnancy.
© 2025 The Author(s). Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Figures
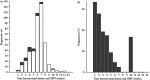
 ) and discordant‐positive (
) and discordant‐positive ( ) non‐invasive prenatal testing (NIPT) results (a) and percentage of NIPT results that were discordant (b), per weekly interval between fetal demise and NIPT in vanishing‐twin pregnancies.
) non‐invasive prenatal testing (NIPT) results (a) and percentage of NIPT results that were discordant (b), per weekly interval between fetal demise and NIPT in vanishing‐twin pregnancies.
 ) and discordant‐positive (
) and discordant‐positive ( ) non‐invasive prenatal testing (NIPT) results per gestational week in vanishing‐twin pregnancies.
) non‐invasive prenatal testing (NIPT) results per gestational week in vanishing‐twin pregnancies.References
-
- Van Den Bogaert K, Lannoo L, Brison N, et al. Outcome of publicly funded nationwide first‐tier noninvasive prenatal screening. Genet Med. 2021;23(6):1137‐1142. - PubMed
-
- Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell‐free DNA in maternal blood in screening for aneuploidies: updated meta‐analysis. Ultrasound Obstet Gynecol. 2017;50(3):302‐314. - PubMed
-
- Rose NC, Barrie ES, Malinowski J, et al. Systematic evidence‐based review: the application of noninvasive prenatal screening using cell‐free DNA in general‐risk pregnancies. Review. Gen Med. 2022;24(7):1379‐1391. - PubMed
-
- Landy HJ, Keith LG. The vanishing twin: a review. Hum Reprod Update. 1998;4(2):177‐183. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

