GLP-1R activation restores Gas6-driven efferocytosis in senescent foamy macrophages to promote neural repair
- PMID: 40915106
- PMCID: PMC12446625
- DOI: 10.1016/j.redox.2025.103857
GLP-1R activation restores Gas6-driven efferocytosis in senescent foamy macrophages to promote neural repair
Abstract
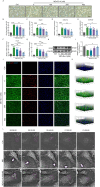
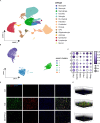
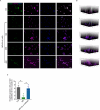
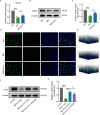
Spinal cord injury (SCI) is a devastating condition characterized by the accumulation of myelin debris (MD), persistent neuroinflammation, and impaired neural regeneration. Although macrophages are pivotal for MD clearance, the impact of excessive MD phagocytosis on macrophage phenotype and function remains poorly understood. Building upon our prior evidence that exendin-4 (Ex-4), a glucagon-like peptide-1 receptor (GLP-1R) agonist, mitigates microglia-driven neuroinflammation post-SCI, this study elucidates the therapeutic efficacy and underlying mechanisms of Ex-4 in alleviating macrophage senescence, restoring efferocytotic capacity, and facilitating neural repair. Employing a T10 contusive SCI model in male C57BL/6 mice, in vivo administration of Ex-4 was combined with macrophage-specific knockdown of growth arrest-specific 6 (Gas6) via AAV-shRNA. Complementary in vitro assays involved bone marrow-derived macrophages (BMDMs) challenged with MD in the presence or absence of Ex-4 or AMP-activated protein kinase (AMPK) inhibition. Cellular senescence and efferocytosis were comprehensively assessed through live-cell imaging, immunofluorescence, senescence-associated β-galactosidase staining, quantitative PCR, and western blotting. Molecular docking and dynamics simulations elucidated GLP-1R-AMPK interactions, corroborated by in vivo validation. Results demonstrate that MD-engulfing macrophages exhibit foam cell-like morphology and upregulated senescence markers, including increased β-galactosidase activity and senescence-associated secretory phenotype, concomitant with diminished efferocytosis via downregulation of the Axl receptor. Senescent macrophages were shown to exacerbate neuronal apoptosis and astrocytic scar formation in co-culture systems. Ex-4 treatment significantly attenuated macrophage senescence, restored efferocytotic function, and reduced neuronal injury and astrocyte activation, effects contingent upon AMPK/Gas6/Axl pathway activation and abrogated by Gas6 knockdown. In vivo, Ex-4 administration enhanced remyelination, axonal regeneration, and functional recovery, while attenuating glial scar formation following SCI. Collectively, these findings identify macrophage senescence induced by excessive MD phagocytosis as a novel pathological contributor to SCI progression and establish Ex-4 as a promising therapeutic agent that restores macrophage homeostasis and promotes neural repair via GLP-1R/AMPK/Gas6/Axl signaling.
Keywords: Efferocytosis; GLP-1R; Gas6; Macrophage senescence; Neuroinflammation; Spinal cord injury.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures










References
-
- Ahuja C.S., et al. Traumatic spinal cord injury. Nat. Rev. Dis. Primers. 2017;3 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

