The role of 3D printing in skeletal muscle-on-a-chip models: Current applications and future potential
- PMID: 40917517
- PMCID: PMC12408411
- DOI: 10.1016/j.mtbio.2025.102222
The role of 3D printing in skeletal muscle-on-a-chip models: Current applications and future potential
Abstract
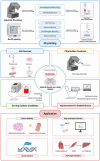
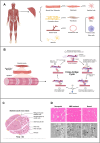
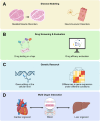
Organ-on-a-chip (OoC) systems can simulate the key functions of human organs, combining microfluidics, cell culture, and biomaterials. 3D printing can be integrated into these technologies to facilitate the construction of OoC models. The high precision and layer-by-layer fabrication process of 3D printing not only enables the creation of complex structures for the microfluidic chip but also improves the cellular microenvironment within the chip by harnessing bioinks for 3D bioprinting. In recent years, OoC models established with 3D printing technology have successfully replicated the functions of various native organs, significantly advancing disease research and drug development. However, due to the complex anatomical structure and unique physiological functions of skeletal muscle, the application of 3D printing in skeletal muscle-on-a-chip (SMoC) models remains relatively limited. Based on existing research on engineered skeletal muscle and OoC, this review discusses the construction of SMoC models by 3D printing to recapitulate the anatomical structure and physiological functions of skeletal muscle. Furthermore, it explores the different applications of 3D printed SMoC models and the future challenges and prospects in this field.
Keywords: 3D printing; Microfluidic technology; Organ-on-a-chip; Skeletal muscle disorders; Skeletal muscle-on-a-chip.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review.Sensors (Basel). 2021 May 10;21(9):3304. doi: 10.3390/s21093304. Sensors (Basel). 2021. PMID: 34068811 Free PMC article.
-
On-chip fabrication and in-flow 3D-printing of microgel constructs: from chip to scaffold materials in one integral process.Biofabrication. 2024 Mar 28;16(2). doi: 10.1088/1758-5090/ad3318. Biofabrication. 2024. PMID: 38471160
-
Innovative organ-on-a-chip platforms for exploring tumorigenesis and therapy in head and neck cancer.J Transl Med. 2025 Jul 16;23(1):798. doi: 10.1186/s12967-025-06824-5. J Transl Med. 2025. PMID: 40671128 Free PMC article. Review.
-
A Comprehensive Review of Organ-on-a-Chip Technology and Its Applications.Biosensors (Basel). 2024 May 1;14(5):225. doi: 10.3390/bios14050225. Biosensors (Basel). 2024. PMID: 38785699 Free PMC article. Review.
-
Innovations in cancer treatment: evaluating drug resistance with lab-on-a-chip technologies.Int J Pharm. 2025 Sep 15;682:125936. doi: 10.1016/j.ijpharm.2025.125936. Epub 2025 Jul 5. Int J Pharm. 2025. PMID: 40623610 Review.
References
LinkOut - more resources
Full Text Sources

