Gut dysbiosis is linked to severe steatosis and enhances its diagnostic performance in MASLD
- PMID: 40917924
- PMCID: PMC12410686
- DOI: 10.1136/egastro-2025-100204
Gut dysbiosis is linked to severe steatosis and enhances its diagnostic performance in MASLD
Abstract
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) is the leading cause of chronic liver disease globally, with rising prevalence linked to metabolic syndrome (MetS). Excessive liver fat accumulation (steatosis) worsens disease progression and MASLD prognosis. Moreover, gut microbiota dysbiosis might promote steatosis, accelerating the disease progression to severe stages. Identifying gut microbiota signatures specific to steatosis severity might improve its diagnosis and inform personalised interventions in MASLD. This study aimed to characterise associations between gut microbiota composition and hepatic steatosis severity in a cohort of patients with MASLD/MetS. Ultimately, we aimed to assess the potential for microbiota features to enhance the diagnosis of severe steatosis.
Methods: A cross-sectional cohort of 61 patients with MetS with extensive clinical history was recruited at different stages of MASLD. Transient elastography was used to evaluate liver fibrosis and steatosis severity. Participants' faecal microbiota were profiled using 16S rRNA gene sequencing. Statistical analyses first identified correlations between microbiota profiles and patients' phenotypes, while disentangling important confounders such as medication. Identified features were then used to build predictive models for diagnosing severe steatosis.
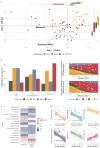
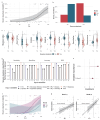
Results: High steatosis severity was distinctly associated with a higher prevalence of the inflammation-associated Bacteroides 2 (Bact2)-enterotype, accompanied by a lower proportion of beneficial commensals (eg, Akkermansia) and a higher proportion of opportunistic bacteria (eg, Streptococcus). Patients harbouring a Bact2-enterotype reached severe steatosis at lower Fatty Liver Index (FLI) thresholds. Using Bact2-carrier status together with FLI in a predictive model significantly improved the classification of severe steatosis (accuracy 90%, receiver operating characteristics 96%) when compared with FLI alone.
Conclusion: Gut microbiota composition and dysbiosis (defined as Bact2-enterotype) are distinctly associated with steatosis severity in MASLD/MetS. Patient stratification by microbiota composition enhances the diagnostic classification of severe steatosis in MASLD, suggesting a potential for personalised interventions in patients with microbiota dysbiosis.
Keywords: Dysbiosis; Fatty Liver; Gastrointestinal Microbiome; Metabolic Syndrome; Metabolic diseases; Microbiota; Non-alcoholic Fatty Liver Disease.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
SV-S and GF have received speaker fees from Ferring and Yakult, respectively. SV-S and GF are listed as inventors on patent WO2019115755A1 ‘A new inflammation-associated, low cell count enterotype’, in the name of VIB VZW, Katholieke Universiteit Leuven, KU Leuven R&D and Vrije Universiteit Brussel. SV-S and GF are credited as inventors on WO2022073973A1 ‘Means and methods to diagnose gut flora dysbiosis and inflammation’, in the name of VIB VZW, Katholieke Universiteit Leuven, KU Leuven R&D and University of Bristol. All other authors declare no competing interests.
Figures
References
LinkOut - more resources
Full Text Sources
