Functional characterization of the α1-adrenoceptor in adult male rat locus coeruleus neurons ex vivo
- PMID: 40918509
- PMCID: PMC12408539
- DOI: 10.3389/fphar.2025.1626019
Functional characterization of the α1-adrenoceptor in adult male rat locus coeruleus neurons ex vivo
Abstract
Introduction: The α1-adrenoceptor (α1AR) is involved in the physiopathology of the central nervous system (CNS), but its function in the adult male rat locus coeruleus (LC) has not been fully studied. We aimed to characterize the role of the α1AR in the regulation of the firing rate (FR) of LC neurons and to describe the signaling pathways involved.
Methods: We measured, through single-unit extracellular recordings of LC neurons from adult male rats were used to measure the effect of adrenergic agonists in the presence and absence of adrenergic antagonists or inhibitors of several signalling pathways.
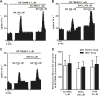
Results: Noradrenaline (NA) (100 µM) and phenylephrine (PE) (100 µM) induced a stimulatory effect in the presence of α2-adrenoceptor (α2AR) antagonist RS 79948 (0.1 µM). The α1AR agonist cirazoline (1-100 µM) also stimulated the FR of LC neurons. The stimulatory effects of NA (100 µM), PE (100 µM), and cirazoline (1 μM and 10 µM) were blocked by α1AR antagonist WB 4101 (0.5 µM). NA (100 µM)-induced stimulation was reduced in the presence of Gi/o protein inactivator pertussis toxin (PTX) (500 ng ml-1) and the transient receptor potential (TRP) channel blocker 2-APB (30 µM), but not by protein kinase C (PKC) inhibitor Go 6976 (1 µM), G protein-activated inward rectifier potassium (GIRK) channel blocker BaCl2 (300 µM), or protein kinase A (PKA) inhibitor H-89 (10 µM). The stimulatory effect of cirazoline was not reduced by any of the tested inhibitors.
Conclusions: From α1AR activation stimulates the FR of adult rat LC neurons through a signaling pathway that involves Gi/o proteins and TRP channels.
Keywords: cirazoline; firing; locus coeruleus; noradrenaline; phenylephrine; rat; slice; α1-adrenoceptor.
Copyright © 2025 Rodilla, Mendiguren and Pineda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Alcántara-Hernández R., Hernández-Méndez A., Romero-Ávila M. T., Alfonzo-Méndez M. A., Pupo A. S., García-Sáinz J. A. (2017). Noradrenaline, oxymetazoline and phorbol myristate acetate induce distinct functional actions and phosphorylation patterns of α1A-adrenergic receptors. Biochim. Biophys. Acta - Mol. Cell Res. 1864, 2378–2388. 10.1016/j.bbamcr.2017.09.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

