Beyond the First Year of Intravitreal Faricimab for Diabetic Macular Oedema: A Single-Centre Experience
- PMID: 40918898
- PMCID: PMC12413623
- DOI: 10.7759/cureus.89526
Beyond the First Year of Intravitreal Faricimab for Diabetic Macular Oedema: A Single-Centre Experience
Abstract
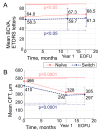
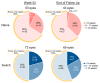
Objective To determine real-world clinical outcomes (including vision, anatomy and durability) of intravitreal faricimab (IVF) in year two (up to mean follow-up of 75 ± 15 weeks, range: 52-103 weeks) of treating diabetic macular oedema (DMO). Secondary objectives included assessing changes in diabetic retinopathy (DR) severity, the incidence of epiretinal proliferation (ERP)/epiretinal membrane (ERM), and safety. Methodology This is a single-centre retrospective observational study. Eligible eyes with ≥52 weeks follow-up, October 2022 to November 2024, were identified and categorised into naïve (no prior anti-vascular endothelial growth factor (anti-VEGF) agents) and switch (≥1 prior anti-VEGF). Descriptive statistics at week 52 (W52) and end of follow-up (EOFU) summarised mean BCVA and CFT change, mean IVF injections and frequency, DR severity change, epiretinal proliferation (ERP)/membrane (ERM) incidence, and safety. Results In total, 158 eyes (66 naïve, 92 switch) of 118 patients were identified, mean follow of 75 weeks. Mean W52 BCVA change was +3.6 (P = 0.04) for naïve and +1.3 letters (P = 0.34) for switch eyes; mean CFT reduction was -141 μm (P < 0.0001) and -115 μm (P < 0.0001), respectively. At EOFU, 27 (63%) naïve eyes and 36 (55%) switch eyes received IVF ≥12 weekly. In switch eyes, the mean W52 IVF interval was 11 weeks versus 7 with prior anti-VEGF agents (P < 0.0001). Of 115 available images, DR severity regressed or was stable in 111 (97%). ERP/ERM was developed in 10/39 (25.6%) naïve and 14/41 (34.1%) switch eyes. No safety outcomes were ever reported. Conclusions Faricimab demonstrated significant structural improvement and durability, with BCVA stability maintained into the second year of treatment. Most eyes showed regression or stability of DR with moderate ERP/ERM incidence.
Keywords: anti-vegf treatment; diabetic macular edema (dme); diabetic macular oedema; faricimab; faricimab intravitreal injenction; intravitreal anti vegf; intravitreal injections; real-world; real-world data.
Copyright © 2025, Tsilegeridis-Legeris et al.
Conflict of interest statement
Human subjects: Informed consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: Theodore Tsilegeridis-Legeris declare(s) Support for attending The Association for Research In Vision and Ophthalmology 2025 Meeting in Salt Lake City, UT, where this work was presented. from Roche UK. Nihal Kenawy declare(s) a grant from Bayer PLC. Nihal Kenawy has received travel grants and advisory board grants from Bayer PLC. Nihal Kenawy declare(s) a grant from AbbVie. Nihal Kenawy has received travel and advisory board grants, as well as financial support from AbbVie for ongoing research. Nihal Kenawy declare(s) a grant from Roche UK. Nihal Kenawy has received travel grants and advisory board grants from Roche UK. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD011599. doi: 10.1002/14651858.CD011599.pub2. Cochrane Database Syst Rev. 2018. PMID: 29669176 Free PMC article.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD007419. doi: 10.1002/14651858.CD007419.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. PMID: 28639415 Free PMC article. Updated.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. Cochrane Database Syst Rev. 2023. PMID: 38275741 Free PMC article.
-
Vitrectomy as an Adjunct to Treat-and-Extend Anti-VEGF Injections for Diabetic Macular Edema: The Vitrectomy in Diabetic Macular Oedema (VIDEO) Randomized Clinical Trial.JAMA Ophthalmol. 2024 Sep 1;142(9):837-844. doi: 10.1001/jamaophthalmol.2024.2777. JAMA Ophthalmol. 2024. PMID: 39115867 Free PMC article. Clinical Trial.
-
Comparative Efficacy, Durability and Safety of Faricimab in the Treatment of Diabetic Macular Edema: A Systematic Literature Review and Network Meta-Analysis.Adv Ther. 2023 Dec;40(12):5204-5221. doi: 10.1007/s12325-023-02675-y. Epub 2023 Sep 26. Adv Ther. 2023. PMID: 37751021 Free PMC article.
References
-
- Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Teo ZL, Tham YC, Yu M, et al. Ophthalmology. 2021;128:1580–1591. - PubMed
-
- Diabetic retinopathy: management and monitoring (NG242) Diabetic Retinopathy. [ Mar; 2025 ]. 2024. https://www.nice.org.uk/guidance/ng242/resources/diabetic-retinopathy-ma... https://www.nice.org.uk/guidance/ng242/resources/diabetic-retinopathy-ma...
-
- Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Wykoff CC, Abreu F, Adamis AP, et al. Lancet. 2022;399:741–755. - PubMed
-
- Faricimab for treating diabetic macular oedema (TA799) Faricimab for treating diabetic macular oedema. [ Mar; 2025 ]. 2022. https://www.nice.org.uk/guidance/ta799/resources/faricimab-for-treating-... https://www.nice.org.uk/guidance/ta799/resources/faricimab-for-treating-...
-
- Khanani AM, Abreu F, Gibson K, et al. [ Aug; 2025 ]. 2025. Four-Year Outcomes of Faricimab in DME: Safety and Efficacy Results From The RHONE-X Long-Term Extension Trial; Presented at the Macula Society 48th Annual Meeting; Charlotte Harbour, FL, 12-15 February 2025.
LinkOut - more resources
Full Text Sources
