A Community Effort to Develop Common Data Elements for Pre-Clinical Spinal Cord Injury Research
- PMID: 40918992
- PMCID: PMC12408885
- DOI: 10.1089/neur.2025.0021
A Community Effort to Develop Common Data Elements for Pre-Clinical Spinal Cord Injury Research
Abstract
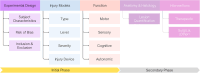
For nearly 350 years, the process of disseminating scientific knowledge has remained largely unchanged. Scientists conduct experiments, analyze the data, and publish their findings in the form of scientific articles. Since the turn of the century, this process has been challenged by numerous open science and data sharing efforts to enhance transparency, reproducibility, and replicability of scientific research. Big data approaches, together with machine learning and artificial intelligence, are frequently used to gain insight into the ever-growing complexity of biological systems and biomedical research. To utilize these approaches and harness the continuously increasing computational power requires data to be both machine readable and, ideally, harmonized across studies. Therein lies the challenge: understanding how to organize and describe data is a critical skill for scientists, yet one that is rarely explicitly taught. Common data elements (CDEs), standardized definitions, and reporting structures for data represent a practical solution to this challenge. With the goal of creating a common language to describe and share pre-clinical spinal cord injury (SCI) research data, the open data commons for SCI, in collaboration with the National Institute of Neurological Disorders and Stroke, kicked off this process with the "Preclinical SCI Common Data Elements (CDE) Workshop," held in conjunction with the National Neurotrauma Symposium in San Francisco, California in June 2024. In this report, we discuss the workshop proceedings, summarize the input provided by the SCI research community, share insights from related CDE efforts, and provide a pragmatic approach to creating CDEs for pre-clinical SCI research.
Keywords: ODC-SCI; common data elements; data management; data sharing; neurotrauma; spinal cord injury.
© The Author(s) 2025. Published by Mary Ann Liebert, Inc.
Figures


References
-
- Cremin CJ, Dash S, Huang X. Big data: Historic advances and emerging trends in biomedical research. Curr Res Biotechnol 2022;4:138–151; doi: 10.1016/j.crbiot.2022.02.004 - DOI
-
- Gandomi A, Haider M. Beyond the hype: Big data concepts, methods, and analytics. Int J Inf Manag 2015;35(2):137–144; doi: 10.1016/j.ijinfomgt.2014.10.007 - DOI
-
- Benson T, Grieve G. Principles of Health Interoperability: FHIR, HL7 and SNOMED CT. Health Information Technology Standards. Springer International Publishing: Cham; 2021; doi: 10.1007/978-3-030-56883-2 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
