Palladium-catalyzed dearomative 1,4-hydroamination
- PMID: 40919097
- PMCID: PMC12410825
- DOI: 10.1016/j.tet.2024.134135
Palladium-catalyzed dearomative 1,4-hydroamination
Abstract
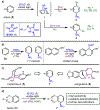
A dearomative 1,4-hydroamination of nonactivated arenes has been developed, using a key arene-arenophile photocycloaddition strategy to disrupt aromaticity. Palladium catalysis with K-Selectride® as a hydride source uniquely enables selective reactivity and provides access to a range of substituted 1,4-cyclohexadienes from aromatic starting materials. We demonstrate a few synthetic applications of this scalable procedure by preparing highly-functionalized small molecules in three to four steps from naphthalene.
Keywords: Arenophiles; Catalysis; Dearomatization; Hydroamination; Palladium.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

