Rare variant genetic landscape of familial chylomicronemia syndrome (FCS) in the United Kingdom
- PMID: 40919303
- PMCID: PMC12409453
- DOI: 10.1016/j.gimo.2025.103445
Rare variant genetic landscape of familial chylomicronemia syndrome (FCS) in the United Kingdom
Abstract
Purpose: Familial chylomicronemia syndrome (FCS) is a rare autosomal recessive disorder. This study aimed to analyze the genotype distribution of FCS-causing genes in the United Kingdom.
Methods: Data were anonymously collated from 2 genetic testing laboratories providing national genetic diagnosis services for severe hypertriglyceridemia in the United Kingdom.
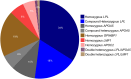
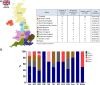
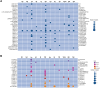
Results: As of December 2023, 880 individuals underwent genetic testing for FCS. The mean (SD) age at the time of genetic testing was 42.5 (15.3) years. After genotyping, 12.9% of the individuals (n = 114) received a genetic diagnosis of FCS. The detection rate of variant-positive multifactorial chylomicronemia syndrome, ie, heterozygous for pathogenic/likely pathogenic (P/LP) variants in 1 of the 5 canonical genes was 11.4% (n = 100).Among 114 genetically proven FCS individuals, 52.6% (n = 60) had biallelic P/LP LPL variants (ie, LPL-FCS), 45.6% (n = 52) had biallelic non-LPL P/LP variants (ie, non-LPL-FCS) and 1.7% (n = 2) individuals were digenic. Among non-LPL-FCS (n = 52), the most common gene implicated was GPIHBP1 (42.3%, n = 22), followed by APOA5 (32.7%, n = 17), LMF1 (13.5%, n = 7) and APOC2 (11.5%, n = 6). Most variant-positive multifactorial chylomicronemia syndrome harbored P/LP variants in LPL (61%) or APOA5 (37%).The geographical distribution of FCS demonstrated regional variability, where the Northwest of England had the highest number of FCS cases per million population. Individuals of European geographic ancestry predominantly had LPL-FCS (60.9%); however, genotype was more diverse in individuals of non-European origin (LPL 47.1%, GPIHBP1 30.9%, APOA5 8.8%, LMF1 7.4%, and APOC2 4.4%). Variants in specific causal genes, GPIHBP1 and LMF1, were predominantly observed in non-European FCS individuals.
Conclusion: The genetic architecture of FCS in the United Kingdom is complex, with a substantial proportion affected by non-LPL FCS-causing genes. It also displays a significant regional and ethnic variations.
Keywords: Autosomal recessive; Chylomicronemia; Genetic variants; Genetics; Hypertriglyceridemia.
© 2025 The Authors.
Conflict of interest statement
Handrean Soran: received personal fees from Amgen, Akcea, Synageva, NAPP, Novartis, Takeda, Sanofi, Pfizer, and Kowa and research grants and donations from Akcea, Pfizer, MSD, Amgen, Genzyme-Sanofi, Synageva, Amryt, Synageva, and Alexion. Anthony S. Wierzbicki: site investigator on trials from Akcea and Regeneron, Royalties from Elsevier for a book on FCS, and is a board member for familial hyperlipidemia group, Europe. Dev Datta: received honoraria for advisory boards from SOBI. Natalie Forrester: received honoraria for presentations from SOBI. Yee Teoh: received speakers fee from Daiichi-Sankyo and Amarin. Paul Downie: received Speaker/Consulting fees from Besins Healthcare, Daiichi-Sankyo, Sanofi, Amgen, Sobi, Novartis, and Amarin. Received financial support for travel and accommodation to attend national/international conferences from Sanofi, Amgen, and Daichii-Sanko Robert A. Hegele: received consulting fees from Acasti, Aegerion, Akcea/Ionis, Amgen, Arrowhead, HLS Therapeutics, Pfizer, Novartis, Regeneron, Sanofi, and UltraGenyx. All other authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

