Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells
- PMID: 40919504
- PMCID: PMC12406528
- DOI: 10.1016/j.mrl.2023.04.003
Using NMR-detected hydrogen-deuterium exchange to quantify protein stability in cosolutes, under crowded conditions in vitro and in cells
Abstract
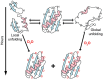
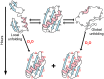
We review the use of nuclear magnetic resonance (NMR) spectroscopy to assess the exchange of amide protons for deuterons (HDX) in efforts to understand how high concentration of cosolutes, especially macromolecules, affect the equilibrium thermodynamics of protein stability. HDX NMR is the only method that can routinely provide such data at the level of individual amino acids. We begin by discussing the properties of the protein systems required to yield equilibrium thermodynamic data and then review publications using osmolytes, sugars, denaturants, synthetic polymers, proteins, cytoplasm and in cells.
Keywords: Amide proton exchange; Cosolutes; Crowding; Equilibrium thermodynamics; Macromolecular; Osmolytes; Protein stability.
© 2023 The Authors.
Conflict of interest statement
Gary J. Pielak is an editorial board member of MRL but was not involved in the editorial review or the decision to publish this article. All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Theillet F.-X., Binolfi A., Frembgen-Kesner T., Hingorani K., Sarkar M., Kyne C., Li C., Crowley P., Gierasch L., Pielak G.J., Elcock A., Gershenson A., Selenko P. Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs) Chem. Rev. 2014;114:6661–6714. - PMC - PubMed
-
- Guseman A.J., Pielak G.J. In: In-cell NMR Spectroscopy: from Molecular Sciences to Cell Biology. Ito Y., Dötsch V., Shirakawa M., editors. The Royal Society of Chemistry; 2020. Protein stability and weak intracellular interactions; pp. 188–206.
-
- Minton A.P. Excluded volume as a determinant of macromolecular structure and reactivity. Biopolymers. 1981;20:2093–2120.
-
- Zimmerman S.B., Trach S.O. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991;222:599–620. - PubMed
Publication types
LinkOut - more resources
Full Text Sources