Pain, Agitation, Delirium, and Iatrogenic Withdrawal Syndrome Management in Children Who Are Critically Ill: Protocol for a European Clinical Practice Guideline Using the Grading of Recommendations Assessment, Development, and Evaluation Approach
- PMID: 40920438
- PMCID: PMC12455155
- DOI: 10.2196/67930
Pain, Agitation, Delirium, and Iatrogenic Withdrawal Syndrome Management in Children Who Are Critically Ill: Protocol for a European Clinical Practice Guideline Using the Grading of Recommendations Assessment, Development, and Evaluation Approach
Abstract
Background: In pediatric intensive care units, pain, sedation, delirium, and iatrogenic withdrawal syndrome (IWS) must be managed as interrelated conditions. Although clinical practice guidelines (CPGs) exist, new evidence needs to be incorporated, gaps in recommendations addressed, and recommendations adapted to the European context.
Objective: This protocol describes the development of the first patient- and family-informed European guideline for managing pain, sedation, delirium, and IWS by the European Society of Paediatric and Neonatal Intensive Care.
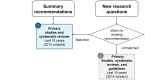
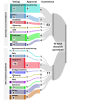
Methods: This guideline will follow the Grading of Recommendations Assessment, Development, and Evaluation ADOLOPMENT approach across seven phases: (1) setup-establish 3 groups, namely a steering committee, development panel (experts and patient and family partners), and patient and family partner advisory panel, to define guideline scope through voting and consensus; (2) preparation-vote on 30 summary recommendations compiled from existing CPGs of medium quality or above; prioritize new research questions; update the search for CPGs to match new research questions with recommendations using population, intervention, comparator, and outcome elements; prioritize outcomes for effectiveness questions using a 9-point Likert scale; with validation from patient and family partners; (3) evidence identification, analysis, and data extraction-develop individualized search strategies for each research question (2 independent appraisers will select and appraise studies and conduct data extraction); (4) evidence synthesis-expert pairs will summarize findings in evidence profiles and evidence-to-decision (EtD) frameworks (in the absence of evidence, the expert panel will be surveyed to assess current practices); (5) guideline development-expert pairs will draft recommendations, then topic-specific subgroups will reach consensus before full development panel voting (>80% approval needed; subgroups will determine the need for additional supporting content); (6) review-conduct internal, society-level, and external international expert reviews using surveys with Likert scales and open-ended comments; and (7) issue and update-publish the guideline and monitor literature to assess the need for updates before 5 years.
Results: In phase 1, a total of 21 clinical experts and 17 patient and family partners were recruited, and the guideline scope was finalized with 80% to 100% agreement. In phase 2, a total of 23 summary recommendations and 17 new research questions (total=40) were selected. The updated CPG search identified 2 low-quality CPGs, which were excluded from recommendation matching. Of the 17 new research questions, 4 matched existing recommendations. Of the 3 effectiveness questions, one had 7 prioritized outcomes, whereas two had 9 outcomes for inclusion in EtD frameworks. The final CPG is expected by spring 2026, with search strategies, EtD frameworks, and recommendations included.
Conclusions: This protocol ensures a transparent Grading of Recommendations Assessment, Development, and Evaluation-based development process, leading to a trustworthy and credible guideline tailored to the European context for managing pain, sedation, delirium, and IWS in children who are critically ill.
International registered report identifier (irrid): DERR1-10.2196/67930.
Keywords: assessment; comfort; critical care; pediatric intensive care; treatment.
©Ibo MacDonald, Alexia Cavin-Trombert, Cécile Jaques, Gwenaëlle De Clifford-Faugère, Pieter A De Cock, Saskia N de Wildt, Dmytro Dmytriiev, Juliane Engel, Paola Claudia Fazio, Sylvia George, Isabelle Goyer, Anna Harðardóttir, Julia Harris, Klára Horváth, Erwin Ista, Santiago Mencía, Tuuli Metsvaht, Maria Cristina Mondardini, Mehdi Oualha, Maria-Helena Perez, Krzysztof Pietrzkiewicz, Francesca Sperotto, Benjamin Wyness, Nilüfer Yalındağ, Angela Amigoni, Anne-Sylvie Ramelet. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 08.09.2025.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures



References
-
- Kiesel LM, Bertsche A, Kiess W, Siekmeyer M, Bertsche T, Neininger MP. Drug-Drug Interactions Involving High-Alert Medications that Lead to Interaction-Associated Symptoms in Pediatric Intensive Care Patients: A Retrospective Study. Paediatr Drugs. 2024 Sep;26(5):619–629. doi: 10.1007/s40272-024-00641-x.10.1007/s40272-024-00641-x - DOI - PMC - PubMed
-
- Egbuta C, Mason KP. Current State of Analgesia and Sedation in the Pediatric Intensive Care Unit. J Clin Med. 2021 Apr 23;10(9):1–26. doi: 10.3390/jcm10091847. https://www.mdpi.com/resolver?pii=jcm10091847 jcm10091847 - DOI - PMC - PubMed
-
- Shajan N, Sharma M, Kaur G. Sedation in pediatric intensive care unit and its impact on outcomes of ventilated children: a prospective observational study. Gaz Egypt Paediatr Assoc. 2023;71(1):41–46. https://epag.springeropen.com/articles/10.1186/s43054-023-00191-w#citeas - DOI
-
- Best KM, Wypij D, Asaro LA, Curley MA, Randomized Evaluation of Sedation Titration For Respiratory Failure Study Investigators Patient, process, and system predictors of iatrogenic withdrawal syndrome in critically ill children. Crit Care Med. 2017 Jan;45(1):e7–15. doi: 10.1097/CCM.0000000000001953. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

