Atomistic simulation of voltage activation of a truncated BK channel
- PMID: 40920564
- PMCID: PMC12416887
- DOI: 10.7554/eLife.105895
Atomistic simulation of voltage activation of a truncated BK channel
Abstract
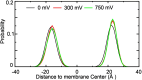
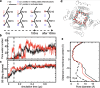
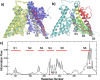
Voltage-dependence gating of ion channels underlies numerous physiological and pathophysiological processes, and disruption of normal voltage gating is the cause of many channelopathies. Here, long timescale atomistic simulations were performed to directly probe voltage-induced gating transitions of the big potassium (BK) channels, where the voltage sensor domain (VSD) movement has been suggested to be distinct from that of canonical Kv channels but remains poorly understood. Using a Core-MT construct without the gating ring, multiple voltage activation transitions were observed at 750 mV, allowing detailed analysis of the activated state of BK VSD and key mechanistic features. Even though the S4 helix remains the principal voltage sensor in BK, its vertical displacement is only ~3 Å and accompanied by significant lateral movements. The nature of the predicted VSD movement is in strong agreement with recent Cryo-EM structural studies of mutant BK channels with constitutively activated VSD. Free energy analysis based on the predicted activation transition yielded a total gating charge of 0.44 e per VSD, consistent with the experimental range of 0.48-0.65 e. We further show that the ability of modest physical movements with a small total gating charge to drive effective voltage gating of BK can be attributed to large gradients in the local electric field as reshaped by the protein. Furthermore, the S4 movement is coupled to the pore opening through a non-canonical pathway that involves the tightly packed S4-S5-S6 interface. These distinct mechanistic features may be relevant to voltage gating of other ion channels where VSDs are not domain-swapped with respect to the pore-gate domain.
Keywords: computational biology; conformational transition; electric field reshaping; free energy; gating charge; hydrophobic gating; molecular biophysics; none; pore-sensor coupling; structural biology; systems biology.
© 2025, Jia and Chen.
Conflict of interest statement
ZJ, JC No competing interests declared
Figures
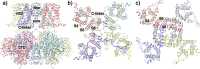









Update of
-
Atomistic Simulation of Voltage Activation of a Truncated BK Channel.bioRxiv [Preprint]. 2025 Jun 14:2025.01.08.631907. doi: 10.1101/2025.01.08.631907. bioRxiv. 2025. Update in: Elife. 2025 Sep 08;14:RP105895. doi: 10.7554/eLife.105895. PMID: 40661448 Free PMC article. Updated. Preprint.
References
-
- Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

