Randomized clinical efficacy and safety study of peltopepimut-S plus cemiplimab compared to cemiplimab alone in patients with recurrent/metastatic HPV16-positive head and neck cancer
- PMID: 40921630
- PMCID: PMC12421166
- DOI: 10.1136/jitc-2025-012555
Randomized clinical efficacy and safety study of peltopepimut-S plus cemiplimab compared to cemiplimab alone in patients with recurrent/metastatic HPV16-positive head and neck cancer
Abstract
Background: Peltopepimut-S is a therapeutic vaccine, which induces specific expansion of both CD4+helper and CD8+cytotoxic T-cells against human papillomavirus type 16 (HPV16) E6/E7 oncoproteins.
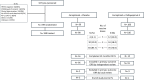
Patients and methods: In a randomized phase 2 trial, we evaluated the efficacy and safety of peltopepimut-S plus cemiplimab compared with cemiplimab alone as first-line or second-line therapy in recurrent/metastatic HPV16-positive head and neck cancer. The primary efficacy endpoint was the objective response rate (ORR) by an independent review (Response Evaluation Criteria in Solid Tumors version 1.1, RECIST v1.1), while the primary safety endpoint was frequency and severity of adverse events. Secondary endpoints included progression-free survival (PFS) and overall survival (OS).
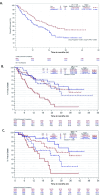
Results: Overall, 198 anti-programmed cell death protein-1 therapy-naïve patients with confirmed HPV16-positive recurrent/metastatic oropharyngeal cancer were randomized to receive cemiplimab plus peltopepimut-S (n=100) or placebo (n=99). The trial did not meet its primary objective (ORR, 25.3% in peltopepimut-S arm vs 22.9% in placebo arm; p=0.735). The median OS (mOS) and PFS in the placebo arm were unexpectedly longer than in the peltopepimut-S arm (26.9 vs 15.8 months) and (20.3 vs 5.5 months), respectively. In predefined exploratory analyses this was associated with an excess death rate from progressive disease in patients with pre-treatment programmed death-ligand 1 (PD-L1) combined positive score (CPS) <20. In contrast, patients with CPS ≥20 had a higher ORR of 51.7% (95% CI, 32.5% to 70.6%) vs 25.8% (95% CI, 11.9% to 44.6%) and a longer mOS in the peltopepimut-S arm (34.8 vs 28.8 months) compared with the placebo arm, respectively. If patients had received all three vaccine or placebo doses, the ORR in patients with CPS ≥20 was 70.0% (95% CI, 45.7% to 88.1%) vs 29.2% (95% CI, 12.6% to 51.1%) in the peltopepimut-S arm and placebo arms, respectively, associated with mOS not reached (after 42 months) versus 23.3 months. The addition of peltopepimut-S to cemiplimab did not increase cemiplimab's toxicity.
Conclusion: Adding peltopepimut-S to cemiplimab did not improve ORR and worsened mOS in the primary analysis. Divergent outcomes were seen in patients with pretreatment PD-L1 CPS <20 (worse ORR and mOS compared with placebo) and CPS ≥20 (higher ORR and longer mOS compared with placebo) values. Future drug development is justifiable in the CPS ≥20 patient population.
Trial registration number: NCT03669718.
Keywords: Combination therapy; Head and Neck Cancer; Immune Checkpoint Inhibitor; Vaccine; Viral-specific T cells.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: KJH, SV, A-SW, LH and CJMM were salaried employees of ISA Pharmaceuticals and author Fury of Regeneron at the time these studies were conducted. ISA and Regeneron sponsored this study together as mentioned elsewhere. After completion of this study ISA Pharmaceuticals went bankrupt. Author CJMM together with a partner not involved in the study bought the peltopepimut-S asset from the bankruptcy lawyers and hopes to further clinically develop this therapeutic vaccine.
Figures
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clinicians. 2021;71:209–49. doi: 10.3322/caac.21660. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
