Effect of nasogastric versus orogastric tube placement on ventilator-associated pneumonia incidence in critically ill patients: a study protocol for a cluster randomised crossover trial in 16 intensive care units in France (SONG trial)
- PMID: 40921641
- PMCID: PMC12421159
- DOI: 10.1136/bmjopen-2025-099840
Effect of nasogastric versus orogastric tube placement on ventilator-associated pneumonia incidence in critically ill patients: a study protocol for a cluster randomised crossover trial in 16 intensive care units in France (SONG trial)
Abstract
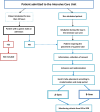
Introduction: Patients in intensive care units (ICUs) frequently require mechanical ventilation, with approximately half needing invasive ventilation through an orotracheal tube. For these patients, gastric tube (GT) insertion is routinely performed to administer nutrition and medications or to drain gastric contents. The insertion route (oral or nasal) may affect the incidence of ventilator-associated pneumonia (VAP), a significant ICU care complication. This study aims to compare the impact of oral versus nasal GT insertion on the incidence of VAP in intubated ICU patients.
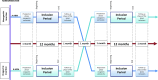
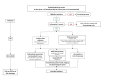
Methods and analysis: The SONG trial (NCT05915663) is a multicentre, open-label, two-period, two-intervention, cluster randomised crossover superiority trial. 16 French ICUs will participate. ICUs will be randomised to periods of nasogastric or orogastric tube placement. The trial includes a practice standardisation period, followed by two 12-month inclusion periods separated by a monitoring and washout period. The primary endpoint is the incidence rate of VAP at day 28, confirmed by three independent physicians. Secondary endpoints include the ease of GT insertion, measured by the number of attempts.
Ethics and dissemination: This study received approval from a central ethical review board on 12 April 2024 (CPP Sud-est VI, registration number 23.00943.000175). Patients are included after informed consent or, when not possible, from next of kin. If none are available, the investigator will proceed with emergency inclusion, following French law. When consent is initially obtained from the next of kin or through emergency inclusion, the investigator will seek consent from the patient as soon as possible. Data will be anonymised and patient confidentiality maintained. Results will be published in peer-reviewed journals and presented at scientific meetings.
Trial registration number: NCT05915663.
Keywords: INTENSIVE & CRITICAL CARE; Nursing Care; Respiratory infections.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: The SONG trial is an investigator-initiated trial funded by the French Ministry of Health in 2020 from a national hospital clinical research programme (Programme Hospitalier de Recherche Infirmière et Paramédicale 2020: PHRIP-20-0278). A scientific committee including JS, CG, JV, SG and JC-C conceived, drafted and wrote the project. All other authors have no competing interests.
Figures
References
-
- Décret n° 2004-802 du 29 juillet 2004 relatif aux parties IV et V (dispositions réglementaires) du code de la santé publique et modifiant certaines dispositions de ce code Les dispositions réglementaires des parties IV et V du code de la santé publique font l’objet d’une publication spéciale annexée au Journal officiel de ce jour (voir à la fin du sommaire). - Légifrance. [17-Oct-2024]. https://www.legifrance.gouv.fr/jorf/id/JORFTEXT000000787339 Available. Accessed.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous