Distinct roles for B cell-derived LTα3 and LTα1β2 in TNF-mediated ileitis
- PMID: 40921841
- PMCID: PMC12479355
- DOI: 10.1038/s41590-025-02263-y
Distinct roles for B cell-derived LTα3 and LTα1β2 in TNF-mediated ileitis
Abstract
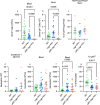
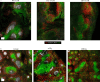
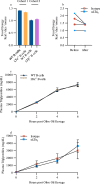
Crohn's disease pathology is modeled in TNFΔARE+/- mice that overproduce tumor necrosis factor (TNF) to drive disease through TNF receptors. An alternative ligand for TNF receptors, soluble LTα3, is produced by B cells, but has received scarce attention because LTα also partners with LTβ to generate membrane-tethered LTαβ2 that promotes tertiary lymphoid tissue-another feature of Crohn's disease. We hypothesized that B cell-derived LTαβ2 would critically affect ileitis in TNFΔARE+/- mice. However, whereas deleting LTβ in B cells was essential for tertiary lymphoid tissue, disease pathology was minimally affected. By contrast, loss of B cell-derived LTα increased intestinal permeability, shrunk the pool of IgA+ ileal plasma cells, elevated cytokines and prompted weight loss, including loss of muscle mass-a systemic feature of Crohn's disease. Neutralizing antibodies to LTα3 strongly augmented the cachexic-like effects of TNF. Thus, B cell-produced LTαβ2 and LTα3 have distinct roles in ileitis, with the role of LTα3 unexpectedly protective through counterbalancing TNF.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.J.R. and R.S.C. receive royalties from the ADAPT-3D method for whole-mount imaging used in this study. The other authors declare no competing interests.
Figures
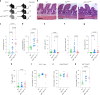
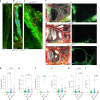
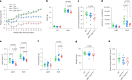

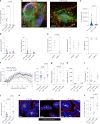






Update of
-
Distinct roles for LTalpha3 and LTalpha1beta2 produced by B cells contribute to their multi-faceted impact on ileitis.Res Sq [Preprint]. 2024 Feb 26:rs.3.rs-3962916. doi: 10.21203/rs.3.rs-3962916/v1. Res Sq. 2024. Update in: Nat Immunol. 2025 Oct;26(10):1781-1793. doi: 10.1038/s41590-025-02263-y. PMID: 38464070 Free PMC article. Updated. Preprint.
References
-
- Torres, J., Mehandru, S., Colombel, J. F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet389, 1741–1755 (2017). - PubMed
-
- Crohn, B. B. & Janowitz, H. D. Reflections on regional ileitis, twenty years later. J. Am. Med. Assoc.156, 1221–1225 (1954). - PubMed
-
- Warren, S. & Sommers, S. C. Pathology of regional ileitis and ulcerative colitis. J. Am. Med. Assoc.154, 189–193 (1954). - PubMed
MeSH terms
Substances
Grants and funding
- 938100/Crohn's and Colitis Foundation (Crohn's & Colitis Foundation)
- R01 AI168044/AI/NIAID NIH HHS/United States
- R37 AI049653/AI/NIAID NIH HHS/United States
- P30 AR073752/AR/NIAMS NIH HHS/United States
- T32 DK077653/DK/NIDDK NIH HHS/United States
- P30 AR074992/AR/NIAMS NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- T32 AI007163/AI/NIAID NIH HHS/United States
- T32DK077653/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- RP220470/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- F30CA281124/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
- DP1DK130660/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- 1019648/Burroughs Wellcome Fund (BWF)
- DP5028125/U.S. Department of Health & Human Services | NIH | NIH Office of the Director (OD)
- T32 DK007130/DK/NIDDK NIH HHS/United States
- R01AI168044/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- DP1 DK130660/DK/NIDDK NIH HHS/United States
- T32 AR007279/AR/NIAMS NIH HHS/United States
- U01 AI163064/AI/NIAID NIH HHS/United States
- P30 DK097948/DK/NIDDK NIH HHS/United States
- P25-01816/American Gastroenterological Association (AGA)
- P30 CA091842/CA/NCI NIH HHS/United States
- F30 CA281124/CA/NCI NIH HHS/United States
- T32 AR060719/AR/NIAMS NIH HHS/United States
- T32DK007130/U.S. Department of Health & Human Services | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (National Institute of Diabetes & Digestive & Kidney Diseases)
- K00 CA264434/CA/NCI NIH HHS/United States
- R01 DK119147/DK/NIDDK NIH HHS/United States
- R03 AI068044/AI/NIAID NIH HHS/United States
- K00CA264434/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- U01AI163064/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P30 DK052574/DK/NIDDK NIH HHS/United States
- T32AI007163/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P01 AG078106/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

