Effect of Empagliflozin on the plasma lipidome in patients with type 2 diabetes mellitus: results from the EmDia clinical trial
- PMID: 40922034
- PMCID: PMC12418620
- DOI: 10.1186/s12933-025-02916-0
Effect of Empagliflozin on the plasma lipidome in patients with type 2 diabetes mellitus: results from the EmDia clinical trial
Abstract
Background: Sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as Empagliflozin, are antidiabetic drugs that reduce glucose levels and have emerged as a promising therapy for patients with heart failure (HF), although the exact molecular mechanisms underlying their cardioprotective effects remain to be fully elucidated. The EmDia study, a randomized, double-blind trial conducted at the University Medical Center of Mainz, has confirmed the beneficial effects of Empagliflozin in HF patients after both one and twelve weeks of treatment. In this work, we aimed to assess whether changes in lipid profiles driven by Empagliflozin use in HF patients in the EmDia trial could assist in gaining a better understanding of its cardioprotective mechanisms.
Methods: Lipid analysis of blood plasma from 144 patients from the EmDia trial was conducted using 4D-LC-TIMS/IMS lipidomics. Lipid signatures after treatment for one and twelve weeks, respectively, were obtained with sparse group LASSO regularized regression models. Linear regression models were employed to highlight associations between significantly changed clinical traits and lipids.
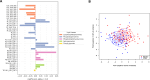
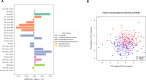
Results: The lipid signatures after one week of treatment consisted of 37 lipids from the lipid groups lysophosphatidylcholine (LPC), phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and triacylglycerol (TG). After twelve weeks, the signature comprised 24 lipids from the same five lipid groups, along with Ceramides (Cer). Three of five lipids altered at both time points showed consistent directional trends. Empagliflozin treatment led to significant alterations in the lipidome, including increases in both beneficial lipids, such as LPCs, and potentially harmful species, notably ceramides, which have been implicated in lipotoxicity and cardiovascular risk.
Conclusion: This study identified distinct lipid signatures associated with Empagliflozin treatment after both one and twelve weeks, respectively, with five lipids overlapping between signatures and three with consistent directions, revealing that some of the beneficial effects of Empagliflozin could be through lipid modulation. Notably, Empagliflozin-modulated lipids associated with changes in clinical traits and lipid-specific profiles among clinical subgroups were observed. However, challenges remain in establishing direct associations between individual lipids and clinical outcomes. Future research integrating lipidomics data with other omics datasets could provide a more comprehensive understanding of the identified lipid signatures and their potential roles in health and diseases.
Trial registration: ClinicalTrials.gov; NCT02932436. Registration date, 2016/10/13.
Keywords: Clinical trial; Empagliflozin; HFpEF; Lipidomics; SGLT2i.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval consent to participate: The local data protection officer and the responsible ethics committee approved the study protocol [ref. no. 2018-13064] prior to study initiation. All study participants provided written informed consent prior to study enrolment. The Declaration of Helsinki [46] and the recommendations of good clinical practice and good epidemiological practice were followed in all study procedures. Consent for publication: Not applicable. Competing interests: Philipp S. Wild reports grants from Bayer AG; non-financial grants from Philips Medical Systems; grants and consulting fees from Boehringer Ingelheim, Novartis AG, Sanofi-Aventis GmbH, and Daiichi Sankyo Europe GmbH; grants and consulting and lecturing fees from Bayer Healthcare Pharmaceuticals; lecturing fees from Pfizer Inc. and Bristol Myers Squibb; consulting fees from AstraZeneca plc; consulting fees and non-financial support from DiaSorin; and non-financial support from I.E.M. Gregor Buch was employed solely at the University Medical Center Mainz during the analysis period and is currently employed by Boehringer Ingelheim. This work was not sponsored by Boehringer Ingelheim and the views of the manuscript do not represent the views of Boehringer Ingelheim. Rights and permissions: Open Access, this article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third-party material in this article are included in the article's Creative Commons license unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Figures

References
-
- Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1141–56. - PubMed
-
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015. 10.1056/NEJMoa1504720. - PubMed
-
- Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. 2022;108:1342–50. - PubMed
-
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

