Amivantamab Plus Chemotherapy in Japanese Patients With EGFR Exon 20 Insertions NSCLC
- PMID: 40922529
- PMCID: PMC12580869
- DOI: 10.1111/cas.70180
Amivantamab Plus Chemotherapy in Japanese Patients With EGFR Exon 20 Insertions NSCLC
Abstract
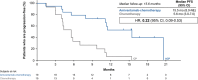
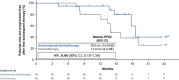
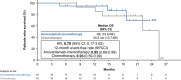
Epidermal growth factor receptor (EGFR) exon 20 insertion mutations (EGFR Exon 20ins) are the third most common mutations in non-small cell lung cancer (NSCLC) and are associated with a poorer prognosis and resistance to conventional EGFR-tyrosine kinase inhibitors. This subpopulation analysis of the open-label phase 3 trial (PAPILLION) evaluates the efficacy and safety of amivantamab-chemotherapy versus chemotherapy among Japanese patients with locally advanced or metastatic NSCLC with EGFR Exon 20ins mutation (ClinicalTrials.gov, NCT04538664). Patients were randomized 1:1 to either intravenous amivantamab plus carboplatin/pemetrexed chemotherapy or chemotherapy alone. The primary endpoint was progression-free survival (PFS) by blinded independent central review; the secondary endpoints included objective response rate, duration of response, PFS after first subsequent therapy, overall survival, and safety. The overall population (n = 308) included 34 Japanese patients (amivantamab-chemotherapy, n = 19; chemotherapy, n = 15). Median PFS was 15.5 (95% CI 8.0, NE) months with amivantamab-chemotherapy compared with 5.6 (95% CI 3.0, 7.0) months (HR = 0.22 [0.09, 0.53]) for chemotherapy alone. Improvements in secondary endpoints were also greater in the amivantamab-chemotherapy arm than the chemotherapy arm. The predominant adverse events associated with amivantamab-chemotherapy were reversible hematologic and EGFR-related toxic effects; 2 of 19 patients discontinued all study agents due to treatment-emergent adverse events. Efficacy and safety results in this Japanese subpopulation were consistent with those in the overall population and support the first-line use of amivantamab-chemotherapy in this setting. Early identification of patients with EGFR Exon 20ins mutations, preferably with more sensitive next-generation sequencing-based methods, is important to ensure appropriate patient access to amivantamab-chemotherapy. Trial Registration: ClinicalTrials.gov, NCT04538664.
Keywords: amivantamab; egfr exon20 insertion; epidermal growth factor receptor; non‐small cell lung cancer; progression‐free survival.
© 2025 Janssen Pharmaceutical K.K. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
M.O. has received research funds provided directly under contracts by a single company or for‐profit organization or by a nonprofit organization funded by the Company with a total value of 1 million yen or more in direct cost from AbbVie Inc., M.S.D. K.K., Parexel International Corporation, Janssen Pharmaceutical K.K., Pfizer Japan Inc., Kaneka Medix Corporation, and Gilead Sciences Inc. O.H. has received research funds provided directly under contracts by a single company or for‐profit organization or by a nonprofit organization funded by the Company with a total value of 1 million yen or more in direct cost from Matsusaka Municipal Hospital. H.T. has received lecture fees, honoraria, or other fees paid by a single company or for‐profit organization for the time or labor of a researcher engaged for conference attendance (as a lecturer, chairperson, ad hoc advisor, etc.) with a total annual value of 500,000 yen or more from AstraZeneca, Eli Lilly Japan, Chugai Pharmaceutical Co. Ltd., and Ono Pharmaceutical Co. Ltd.; research funds provided directly under contracts by a single company or for‐profit organization, or by a non‐profit organization funded by the Company with a total value of 1 million yen or more in direct cost from AstraZeneca, Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Daiichi Sankyo Company, Limited, M.S.D., Amgen, Janssen Pharmaceutical K.K., and Merck. S.M. has received lecture fees, honoraria, or other fees paid by a single company or for‐profit organization for the time or labor of a researcher engaged for conference attendance (as a lecturer, chairperson, ad hoc advisor, etc.) with a total annual value of 500,000 yen or more from Eli Lilly, Merck Biopharma, Chugai Pharma, Novartis Pharma, Guardant Health, and AstraZeneca; research funds provided directly under contracts by a single company or for‐profit organization, or by a nonprofit organization funded by the Company with a total value of 1 million yen or more in direct cost from Chugai Pharma, Janssen Pharmaceutical K.K., and M.S.D. H.H. has received lecture fees, honoraria, or other fees paid by a single company or for‐profit organization for the time or labor of a researcher engaged for conference attendance (as a lecturer, chairperson, ad hoc advisor, etc.) with a total annual value of 500,000 yen or more from AstraZeneca K.K., Bristol Myers Squibb K.K., Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Daiichi Sankyo Company, Limited; research funds provided directly under contracts by a single company or for‐profit organization, or by a non‐profit organization funded by the Company with a total value of 1 million yen or more in direct cost from A2 Healthcare Corp., IQVIA Services Japan K.K., PRA Health Sciences Inc., SYNEOS HEALTH CLINICAL K.K., MSD K.K., GlaxoSmithKline K.K., Pfizer R&D Japan G.K., Daiichi Sankyo Company, Limited, AbbVie Inc., Astellas Pharma Inc., Amgen Inc., AstraZeneca K.K., Taiho Pharmaceutical Co. Ltd., CMIC Co. Ltd., Chugai Pharmaceutical Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., EPS Corporation, West Japan Oncology Group; scholarship (incentive) endowments provided directly from a single company or for‐profit organization, or research grants provided directly from a private academic support organization with a total annual value of 1 million yen or more from Chugai Pharmaceutical Co. Ltd. M.T., T.A., T.S., and A.Y. are full‐time employees of Janssen Pharmaceutical K.K. T.A., A.B., M.B., R.E.K., and H.W. are full‐time employees of Janssen R&D. Although S.K. was affiliated with The Cancer Institute Hospital of J.F.C.R. and had no conflicts of interest to declare while conducting the research, he is currently an employee of Janssen Pharmaceutical K.K., A.O., T.K., N.W., and H.N. have no conflicts of interest to declare.
Figures


References
-
- Nagasaka M., Goto K., Gomez J., et al., “P50.04 Amivantamab in Combination With Chemotherapy in Patients With Advanced Non‐Small Cell Lung Cancer (NSCLC),” Journal of Thoracic Oncology 16 (2021): S1116, 10.1016/j.jtho.2021.08.532. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

