Trauma community clinical guidance needs: a mixed-methods iterative consensus-building study
- PMID: 40923036
- PMCID: PMC12414228
- DOI: 10.1136/tsaco-2024-001592
Trauma community clinical guidance needs: a mixed-methods iterative consensus-building study
Abstract
Introduction: Developing and implementing trauma clinical guidance is integral to providing quality care to all trauma patients while maintaining a minimum standard of treatment. A mixed-methods novel consensus-building approach was used to identify the current barriers to developing and implementing trauma clinical guidance and highlight the priority areas for change to better support end users.
Methods: As part of year 1 of the Design for Implementation: The Future of Trauma Clinical Guidance and Research Conference Series, preconference participant surveys and hybrid, professionally facilitated, structured dialogue were used to define the ideal future state of trauma clinical guidance development and dissemination. Novel to this context, in-person and virtual "user stories", a form of structured focus group, were generated, and a "minimum viable product" (MVP), a form of brokered dialogue, was developed. Descriptive statistics and thematic analysis were used to evaluate preconference survey and "user story" results.
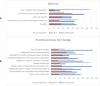
Results: 72 in-person and up to 35 virtual attendees participated. The majority (92%) of in-person attendees and nearly half (48%) of virtual attendees completed the preconference survey. Participants identified barriers along the continuum of clinical guidance development, dissemination, and adoption. Areas for improvement centered around the creation, storage, and use of guidance. Across the survey and user stories, participants expressed the need for clinical guidance that is comprehensive, evidence-based, coordinated, and easily accessible by all clinicians both domestically and abroad. The MVP targeted the risks and objectives to improved guidance. A prominent theme throughout this consensus-building assessment was the imperative for collaboration between professional societies for clinical guidance development and dissemination.
Discussion: Trauma clinical guidance must be current, consolidated, and coordinated with patient-centered outcomes prioritized. Next steps include turning the MVP produced into a prototype and refining it to inform a national redesign of trauma clinical guidance.
Level of evidence: Level III.
Keywords: Clinical; guideline.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
AS, AN, KTG, GZW, ANM and LNL report that funding for the DFI conference was made possible in part by grant 1R13HS028940-01A1 from the Agency for Healthcare Research and Quality (AHRQ) paid to the Coalition for National Trauma Research. The AHRQ grant covered their costs for attending the conference. ANM received financial support from The ReSource, LLC for additional DFI conference support. LNL received support from the American College of Surgeons, Committee on Trauma for meetings and travel. GZW reports support from Sociedad de Cirujanos Generales del Peru Research Group.
Figures


References
-
- Development of a planning tool to guide research dissemination - advances in patient safety: from research to implementation (volume 4: programs, tools, and products) NCBI; https://www.ncbi.nlm.nih.gov/books/NBK20603/ Available. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
