Ataxia and cerebellar hypoexcitability in a mouse model of SCN1B-linked Dravet syndrome
- PMID: 40923316
- PMCID: PMC12487675
- DOI: 10.1172/jci.insight.187606
Ataxia and cerebellar hypoexcitability in a mouse model of SCN1B-linked Dravet syndrome
Abstract
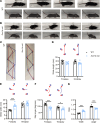
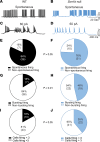
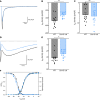
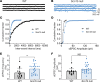
Patients with Dravet syndrome (DS) present with severe, spontaneous seizures and ataxia. While most patients with DS have variants in the sodium channel Nav1.1 α subunit gene, SCN1A, variants in the sodium channel β1 subunit gene, SCN1B, are also linked to DS. Scn1b null mice model DS, with spontaneous generalized seizures that start in the second week of life. In Scn1b null cerebellum, neuronal pathfinding is severely altered, and Purkinje cells (PCs) and granule neurons have altered excitability. Here, we show that Scn1b null mice are ataxic. Expression of β1 protein in WT cerebellum, assessed using a CRISPR transgenic mouse model containing an in-frame V5 epitope tag at the β1 C-terminus, is widespread. Scn1b null PCs and interneurons in cerebellar slices have increased thresholds for action potential initiation and decreased repetitive firing frequency compared with WT. Scn1b null PCs have reduced transient and resurgent sodium current densities. We propose that reduced PC excitability underlies the ataxic phenotype of Scn1b mice. In addition, because cerebellar output to other areas of the brain can result in termination of seizures, we propose that PC hypoexcitability exacerbates the severe phenotype of this mouse model.
Keywords: Epilepsy; Genetics; Mouse models; Neuroscience; Sodium channels.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

