Dendritic cells: understanding ontogeny, subsets, functions, and their clinical applications
- PMID: 40924040
- PMCID: PMC12420571
- DOI: 10.1186/s43556-025-00300-8
Dendritic cells: understanding ontogeny, subsets, functions, and their clinical applications
Abstract
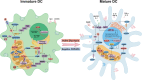
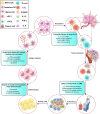
Dendritic cells (DCs) play a central role in coordinating immune responses by linking innate and adaptive immunity through their exceptional antigen-presenting capabilities. Recent studies reveal that metabolic reprogramming-especially pathways involving acetyl-coenzyme A (acetyl-CoA)-critically influences DC function in both physiological and pathological contexts. This review consolidates current knowledge on how environmental factors, tumor-derived signals, and intrinsic metabolic pathways collectively regulate DC development, subset differentiation, and functional adaptability. Acetyl-CoA emerges as a dual-function metabolite, serving not only as an energy carrier but also as an epigenetic regulator that controls DC fate via lipid biosynthesis, mitochondrial metabolism, and chromatin modification. In the tumor microenvironment (TME), DCs may experience immune suppression polarization and insufficient T cell activation due to disrupted acetyl-CoA related metabolic pathways. While existing DC-based therapies remain constrained by TME-induced metabolic limitations, emerging approaches that restore acetyl-CoA related metabolic pathways balance show enhanced antitumor efficacy. The review further examines distinct metabolic adaptations among DC subsets and their relevance to autoimmune diseases, infectious immunity, and transplant outcomes. By integrating current research on targeting DC metabolic targets, we outline strategies for developing immunotherapies that target DC metabolic flexibility. Remaining hurdles include tailoring interventions to specific subsets, refining metabolic manipulation techniques, and addressing TME heterogeneity through combination therapies. These findings position acetyl-CoA as a key therapeutic target for recalibrating immunometabolism circuits, with significant implications for DC-focused cancer treatment.
Keywords: Acetyl-CoA; Cancer therapy; Dendritic cells; Immunotherapy; Metabolic reprogramming; Tumor microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: No applicable. Consent for publication: No applicable. Competing interests: The author asserts that no competitive or conflicting interests are present in this article.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
