Context-dependent contribution of peptidyl arginine deiminase 4 (PAD4) to neutrophil extracellular trap formation and liver injury in acute and chronic hepatotoxicant challenge
- PMID: 40924556
- PMCID: PMC12599872
- DOI: 10.1093/toxsci/kfaf123
Context-dependent contribution of peptidyl arginine deiminase 4 (PAD4) to neutrophil extracellular trap formation and liver injury in acute and chronic hepatotoxicant challenge
Abstract
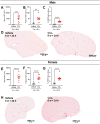
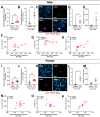
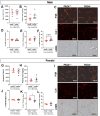
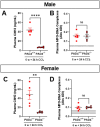
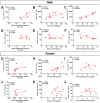
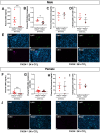
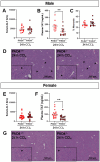
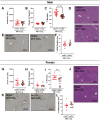
Neutrophils play a complex role in the pathogenesis of chronic liver disease and have been linked to both liver damage and injury resolution. Recent reports propose that neutrophils drive liver injury and fibrosis through the formation of neutrophil extracellular traps (NETs). This study tests the hypothesis that the enzyme peptidyl arginine deiminase-4 (PAD4) drives NET formation and liver fibrosis in experimental chronic liver injury. Wild-type (PAD4+/+) and PAD4-deficient (PAD4-/-) mice were chronically challenged twice weekly with carbon tetrachloride (CCl4, 1 ml/kg, i.p) or vehicle (corn oil) for 6 weeks, and samples were collected 24 h after the final challenge. In separate studies, mice were challenged once, and samples were collected 24 to 48 h later. Circulating NET biomarkers (e.g. myeloperoxidase-DNA complexes) were elevated in chronic CCl4-challenged wild-type mice compared to vehicle, though surprisingly, intrahepatic NETs were rarely observed. In contrast to our hypothesis, PAD4 deficiency did not eliminate circulating NET markers in chronic challenge. Furthermore, PAD4 deficiency did not impact liver fibrosis assessed by picrosirius red labeling or the myofibroblast marker α-smooth muscle actin but caused a modest, sex-specific decrease in hepatic collagen type I immunolabeling. Interestingly, plasma NET biomarkers and intrahepatic NETs were both increased following acute CCl4 challenge in a PAD4-dependent manner. Furthermore, PAD4 deficiency reduced coagulation activity after 24 h and decreased hepatocellular necrosis 48 h after challenge. Our studies ultimately suggest that PAD4 affects liver injury uniquely, depending on the stage of disease and that mechanisms of NET formation may occur independent of PAD4 in chronic liver injury.
Keywords: hepatic; hepatotoxicity; inflammation; liver; pathology.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures


References
-
- Alkhouri N, Morris‐Stiff G, Campbell C, Lopez R, Tamimi TAR, Yerian L, Zein NN, Feldstein AE. 2012. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 32:297–302. - PubMed
-
- Ambrosino P, Tarantino L, Di Minno G, Paternoster M, Graziano V, Petitto M, Nasto A, Di Minno MN. 2017. The risk of venous thromboembolism in patients with cirrhosis. A systematic review and meta-analysis. Thromb Haemost. 117:139–148. - PubMed
-
- Babuta M, Morel C, de Carvalho Ribeiro M, Calenda C, Ortega-Ribera M, Thevkar Nagesh P, Copeland C, Zhuang Y, Wang Y, Cho Y, et al. 2024. Neutrophil extracellular traps activate hepatic stellate cells and monocytes via nlrp3 sensing in alcohol-induced acceleration of mash fibrosis. Gut. 73:1854–1869. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

