Natural history and development of a novel composite endpoint in patients with alcohol-associated hepatitis: Data from a prospective multicenter study
- PMID: 40924794
- PMCID: PMC12672022
- DOI: 10.1097/HEP.0000000000001513
Natural history and development of a novel composite endpoint in patients with alcohol-associated hepatitis: Data from a prospective multicenter study
Abstract
Background aims: The clinical course and outcomes of alcohol-associated hepatitis (AH) remain poorly understood. Major adverse liver outcomes do not capture the added risk of return to drinking. We examined the natural history of AH and developed a composite endpoint using a contemporary observational cohort of AH.
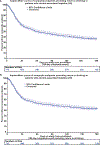
Approach results: A cohort of 1127 participants: 712 AH patients, 256 heavy drinking controls without clinically evident liver disease, and 159 healthy controls, were prospectively followed for 6 months at 8 United States centers as part of the Alcoholic Hepatitis Network (AlcHepNet) consortium. Outcomes included mortality and a composite endpoint (AlcHepNet composite index) that included death, liver transplantation, hepatic decompensation (new onset/worsening ascites, HE, variceal bleeding), liver-related hospital admission, MELD increase ≥5, and return to drinking. Of 712 AH patients (age 45±10.7 y; 59.1% male), 558 (79.0%) had severe and 148 (21.0%) had moderate AH, 232 (32.5%) died, and 86 (12.1%) underwent liver transplantation. Mortality rates in moderate AH and severe AH were 0.7% versus 17.2% (30 d), 3.4% versus 26.5% (90 d), and 8.8% versus 30.5% (180 d), respectively (all p <0.001). Composite liver/alcohol use events were noted in 459 (64.5%) AH patients. Higher MELD score, lower mean arterial pressure, and baseline leukocytosis were associated with higher 90-day mortality in AH (all p <0.05). College education and higher ALP were associated with lower mortality. Heavy drinking controls had low mortality (n=3; 1.2%).
Conclusions: This large observational study showed a high incidence of composite liver and alcohol-use events within 6 months, reiterating the need for early interventions.
Keywords: alcohol-associated hepatitis; composite event; multicenter; outcomes; prospective.
Copyright © 2025 American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflicts of interest. Naga Chalasani has consulting agreements with Madrigal, Zydus, Altimmune, Ipsen, Biomed Fusion, GSK, Pfizer, Akero, and Boston Pharmaceuticals. He receives research support from Exact Sciences, Boehringer-Ingelheim. He has equity in Avant Sante, a contract research organization, and Heligenics, a drug discovery start-up company.
Figures


References
-
- Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, McCullough AJ, Dasarathy S. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res. 2015;39:2085–2094. doi: 10.1111/acer.12887 - DOI - PMC - PubMed
Grants and funding
- R01 AA028190/AA/NIAAA NIH HHS/United States
- R21 AA022742/AA/NIAAA NIH HHS/United States
- U01 AA021890/AA/NIAAA NIH HHS/United States
- U01 AA026980/AA/NIAAA NIH HHS/United States
- U01 AA026976/AA/NIAAA NIH HHS/United States
- K08 AA028794/AA/NIAAA NIH HHS/United States
- R01 DK133905/DK/NIDDK NIH HHS/United States
- R01 DK113196/DK/NIDDK NIH HHS/United States
- P50 AA024333/AA/NIAAA NIH HHS/United States
- I01 CX002219/CX/CSRD VA/United States
- R01 GM119174/GM/NIGMS NIH HHS/United States
- P50 AA024337/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources

