Genomic and morphological characterization of a novel iridovirus, bivalve iridovirus 1 (BiIV1), infecting the common cockle (Cerastoderma edule)
- PMID: 40924942
- PMCID: PMC12452177
- DOI: 10.1099/mgen.0.001494
Genomic and morphological characterization of a novel iridovirus, bivalve iridovirus 1 (BiIV1), infecting the common cockle (Cerastoderma edule)
Abstract
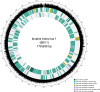
High rates of mortality of the common cockle, Cerastoderma edule, have occurred in the Wash Estuary, UK, since 2008. A previous study linked the mortalities to a novel genotype of Marteilia cocosarum, with a strong correlation between cockle moribundity and the presence of M. cocosarum. Here, we characterize a novel iridovirus, identified by chance during metagenomic sequencing of a gradient purification of Marteilia cells, with the presence also correlated to cockle moribundity. The novel 179,695 bp iridovirus, bivalve iridovirus 1 (BiIV1), encodes 193 predicted ORFs and has a G+C content of 41 mol%. BiIV1 clusters together with other aquatic invertebrate iridoviruses in phylogenetic analyses and has a similar genome size to other invertebrate iridoviruses. Comparative analysis revealed that BiIV1 has lost three genes that were previously thought to be common amongst all iridoviruses but has also gained genes, potentially from horizontal transfer from its bivalve mollusc host(s). Electron microscopy showed 158 nm icosahedral virions present in the haemocytes of cockles, typical of those observed in host tissues infected with viruses of the family Iridoviridae. Prevalence of BiIV1 in moribund cockles was higher than that in apparently healthy cockles at most sites in the Wash Estuary, with up to 100% PCR prevalence in moribund cockles. Our findings provide the first genome for a bivalve-infecting iridovirus and identify a second bivalve-associated iridovirus in publicly available genomic datasets, adding to the knowledge of invertebrate iridovirus genomics and diversity.
Keywords: aquatic animal health; bivalve; cockle; emerging disease; iridovirus.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- Fishery and aquaculture statistics FAO Fisheries and Aquaculture Division. 2022. www.fao.org/fishery/statistics/software/fishstatj/es
-
- Montaudouin X, Arzul I, Cao A, Carballal MJ, Chollet B, et al. Catalogue of Parasites and Diseases of the Common Cockle Cerastoderma Edule - COCKLES PROJECT. Portugal: UA Editora - Universidade de Aveiro; 2021. - DOI
-
- Carrasco N, Hine PM, Durfort M, Andree KB, Malchus N, et al. Marteilia cochillia sp. nov., a new Marteilia species affecting the edible cockle Cerastoderma edule in European waters. Aquaculture. 2013;412–413:223–230. doi: 10.1016/j.aquaculture.2013.07.027. - DOI
-
- Skujina I, Hooper C, Bass D, Feist SW, Bateman KS, et al. Discovery of the parasite Marteilia cocosarum sp. nov. In common cockle (Cerastoderma edule) fisheries in Wales, UK and its comparison with Marteilia cochillia. J Invertebr Pathol. 2022;192:107786. doi: 10.1016/j.jip.2022.107786. - DOI - PubMed
-
- Tidy A, Jessop R, Ward GM, Green MJ, Bateman KS, et al. Characterisation of Marteilia cocosarum in the Wash Estuary, UK, linked to mass mortalities of cockles (Cerastoderma edule), and its relationship to closely related species. Mol Biol. 2025:2025. doi: 10.1101/2025.02.13.638039. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

