Modulation of fibronectin extracellular matrix enhances anti-tumor efficacy of immune checkpoint blockade
- PMID: 40925374
- PMCID: PMC12490255
- DOI: 10.1016/j.xcrm.2025.102322
Modulation of fibronectin extracellular matrix enhances anti-tumor efficacy of immune checkpoint blockade
Abstract
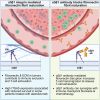
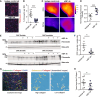
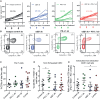
The success of immune checkpoint inhibitors is limited by multiple factors, including poor T cell infiltration and function within tumors, partly due to a dense extracellular matrix (ECM). Here, we investigate modulating the ECM by targeting integrin α5β1, a major fibronectin-binding and organizing integrin, to improve immunotherapy outcomes. Use of a function-blocking murinized α5β1 antibody reduces fibronectin fibril formation, enhances CD8+ T cell transendothelial migration, increases vascular permeability, and decreases vessel-associated collagen. These changes culminate in improving the effectiveness of PD-L1 blockade, alone or with chemotherapy, in the E0771 breast cancer model. Clinically, high integrin alpha 5 (ITGA5) expression correlates with worse survival in patients treated with atezolizumab as monotherapy or combined with chemotherapy or anti-angiogenic therapies in numerous clinical trials. Overall, our studies suggest that ECM-modulating approaches could be used as a future strategy to increase the proportion of patients who respond to immune checkpoint inhibition and other immunotherapies.
Keywords: atezolizumab; cancer; combination; extracellular matrix; fibronectin; integrin a5b1; tumor immunotherapy.
Copyright © 2025 Genentech, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests During the course of these studies, R.S.K. was a scientific advisory board (S.A.B) member of Novelty Nobility (Seoul, Republic of Korea), a consultant for Pharmabcine Inc (Daejeon, Republic of Korea) and a consultant to Eyepoint Pharmaceuticals (USA), and is currently an S.A.B member of OncoHost (Haifa, Israel). W.Y., R.P., R.W., A.G., R.J., and M.C.M. are or were employees of Genentech (a Roche subsidiary), and some hold Roche stocks. W.Y. has issued (US-8962275-B2) and pending patents regarding human integrin α5β1 antibodies.
Figures




References
-
- Blasutig I.M., Farkona S., Buchbinder E.I., Luke J.J., Sharma P., Sznol M. The Phoenix Rises: The Rebirth of Cancer Immunotherapy. Clin. Chem. 2017;63:1190–1195. - PubMed
-
- Adams S., Schmid P., Rugo H.S., Winer E.P., Loirat D., Awada A., Cescon D.W., Iwata H., Campone M., Nanda R., et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:397–404. - PubMed
-
- Emens L.A., Cruz C., Eder J.P., Braiteh F., Chung C., Tolaney S.M., Kuter I., Nanda R., Cassier P.A., Delord J.-P., et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients With Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5:74–82. - PMC - PubMed
-
- Adams S., Loi S., Toppmeyer D., Cescon D.W., De Laurentiis M., Nanda R., Winer E.P., Mukai H., Tamura K., Armstrong A., et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann. Oncol. 2019;30:405–411. - PubMed
-
- Dirix L.Y., Takacs I., Jerusalem G., Nikolinakos P., Arkenau H.-T., Forero-Torres A., Boccia R., Lippman M.E., Somer R., Smakal M., et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018;167:671–686. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

