Time restricted eating and exercise training before and during pregnancy for people with increased risk of gestational diabetes: single centre randomised controlled trial (BEFORE THE BEGINNING)
- PMID: 40925657
- PMCID: PMC12418603
- DOI: 10.1136/bmj-2024-083398
Time restricted eating and exercise training before and during pregnancy for people with increased risk of gestational diabetes: single centre randomised controlled trial (BEFORE THE BEGINNING)
Abstract
Objective: To determine the effect of a prepregnancy lifestyle intervention on glucose tolerance in people at higher risk of gestational diabetes mellitus.
Design: Single centre randomised controlled trial (BEFORE THE BEGINNING).
Setting: University hospital in Trondheim, Norway.
Participants: 167 participants with at least one risk factor for gestational diabetes mellitus who contemplated pregnancy.
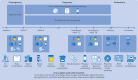
Intervention: The participants were randomly allocated (1:1) to a lifestyle intervention or a standard care control group. The intervention consisted of exercise training and time restricted eating, started before pregnancy and continued throughout pregnancy. Exercise volume was set using a physical activity metric that translates heart rate into a score (personal activity intelligence, PAI), with the goal of ≥100 weekly PAI points. Time restricted eating involved consuming all energy within ≤10 hours/day for at least five days a week.
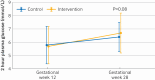
Main outcome measures: Two hour plasma glucose level in an oral glucose tolerance test at gestational week 28. The primary analysis used an intention-to-treat principle.
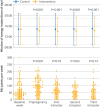
Results: 167 participants were enrolled from 2 October 2020 to 12 May 2023: 84 in the intervention group and 83 in the control group, out of whom 111 became pregnant (56 in intervention group and 55 in control group). One participant in the intervention group was excluded from the analysis because of prepregnancy diabetes. Pregnancy data from one participant in the control group were excluded from the analysis because of twin pregnancy. The intervention had no significant effect on two hour plasma glucose level in an oral glucose tolerance test at gestational week 28 (mean difference 0.48 mmol/L, 95% confidence interval -0.05 to 1.01, P=0.08). In the prepregnancy period, 31/83 participants (37%) in the intervention group adhered to prespecified criteria, whereas 24/55 participants (44%) in the intervention group who became pregnant fulfilled these criteria. During the prepregnancy period, the average eating window was 9.9 hours/day (standard deviation 1.2) and the average number of weekly PAI points was 111 (standard deviation 54), but the adherence to both intervention components decreased during pregnancy.
Conclusions: A combination of time restricted eating and exercise training started before and continued throughout pregnancy had no significant effect on glycaemic control in late pregnancy.
Trial registration: ClinicalTrials.gov NCT04585581.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure at https://www.icmje.org/disclosure-of-interest/ and declare: financial support for the submitted work from the Novo Nordisk Foundation, the Liaison Committee for education, research and innovation in Central Norway, and the Joint Research Committee between St Olav’s Hospital and the Faculty of Medicine and Health Sciences, NTNU. TM has received research grants from the European Commission, the Liaison Committee for education, research and innovation in Central Norway, and the Joint Research Committee between St Olav’s Hospital and the Faculty of Medicine and Health Sciences, NTNU, the Dam foundation, and NTNU Health and Life Sciences strategic area, consultation fees from the University of Stavanger, payment for lectures from the Norwegian Physiotherapist Association, has served as a board member in the European Association of Preventive Cardiology (EAPC), and is a member of the section for Primary Care and Risk Factor Management in EAPC. SLF has received lecture honorary from Sanofi Aventis, payment for Novo Nordisk Norway for a chapter in an insulin guide and for attending meetings, and has participated in the Nordic Acromegaly Advisory Board. The authors declare no financial relationships with any organisations that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- International Diabetes Federation, Brussels, Belgium. IDF Diabetes Atlas 2021 10th edition. Available at: https://diabetesatlas.org/atlas/tenth-edition/. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
