Differences in Learning Strategy Selection and Object Location Memory Impairments in APP/PS1 Mice
- PMID: 40925884
- PMCID: PMC12426418
- DOI: 10.5607/en25013
Differences in Learning Strategy Selection and Object Location Memory Impairments in APP/PS1 Mice
Abstract
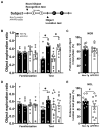
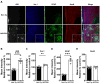
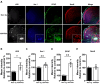
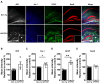
This study investigated the learning strategy preferences of 11-month-old APP/PS1 double transgenic (Tg) mice, a well-established murine model of Alzheimer's disease (AD). APP/PS1 Tg and non-Tg control mice were serially trained in visual and hidden platform tasks in the Morris water maze. APP/PS1 Tg mice performed poorly in visual platform training compared with non-Tg mice but performed as well as non-Tg mice in hidden platform training. Further analysis of their search paths for locating a hidden platform revealed that APP/PS1 Tg mice used more cued/response search patterns than place/spatial search patterns compared with non-Tg mice. Three months later, the object/location recognition memory of APP/PS1 Tg mice was assessed. Although their object recognition memory was intact, their object location memory was impaired. Neuropathological AD features of APP/PS1 transgenic mice were observed in the medial prefrontal cortex, retrosplenial cortex, and hippocampus, key brain regions involved in learning strategy shifts and spatial cognition. These results indicate that distinct search patterns and spatial memory deficits in APP/PS1 Tg mice are key features of AD animal models.
Keywords: APP/PS1; Alzheimer’s disease; Amyloid beta; Cued/response learning; Learning strategy; Object location memory.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

