Anatomical circuits for flexible spatial mapping by single neurons in posterior parietal cortex
- PMID: 40925912
- PMCID: PMC12420791
- DOI: 10.1038/s42003-025-08596-6
Anatomical circuits for flexible spatial mapping by single neurons in posterior parietal cortex
Abstract
Primate lateral intraparietal area (LIP) has been directly linked to perceptual categorization and decision-making. However, the intrinsic LIP circuitry that gives rise to the flexible generation of motor responses to sensory instruction remains unclear. Using retrograde tracers, we delineate two distinct operational compartments based on different intrinsic connectivity patterns of dorsal and ventral LIP. These connections form an anatomical loop with a sensory-like, point-to-point projection from ventral to dorsal LIP and an asymmetric, widespread projection in reverse. In neurophysiological recordings, LIP neurons exhibit motor response fields spatially distinct from their sensory receptive field. Different associations of motor response and receptive fields in single neurons tile visual space. Ventral LIP neurons tend to have motor response fields distant from their sensory receptive fields. This circuit provides the neural substrate to generate the dynamic processes for flexible allocation of attention and motor responses in response to salient or instructive visual input across the visual field.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. All animal procedures were approved by United Kingdom Home Office Licences issued by The Animals in Science Regulation Unit (ASRU).
Figures


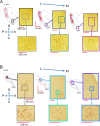
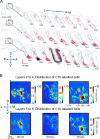

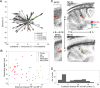

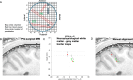
References
-
- Andersen, R. A. & Cui, H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron63, 568–583 (2009). - PubMed
-
- Colby, C. L. & Goldberg, M. E. Space and attention in parietal cortex. Annu. Rev. Neurosci.22, 319–349 (1999). - PubMed
-
- Glimcher, P. W. Decisions, Uncertainty and the Brain. The Science of Neuroeconomics (MIT Press, 2003).
-
- Gold, J. I. & Shadlen, M. N. The neural basis of decision making. Annu. Rev. Neurosci.30, 535–574 (2007). - PubMed
MeSH terms
Grants and funding
- 507082410/Deutsche Forschungsgemeinschaft (German Research Foundation)
- BB/H016902/1/RCUK | Biotechnology and Biological Sciences Research Council (BBSRC)
- 425899996/Deutsche Forschungsgemeinschaft (German Research Foundation)
- WT_/Wellcome Trust/United Kingdom
- 406269671/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources

