Durotaxis is a driver and potential therapeutic target in lung fibrosis and metastatic pancreatic cancer
- PMID: 40925952
- PMCID: PMC12431851
- DOI: 10.1038/s41556-025-01697-8
Durotaxis is a driver and potential therapeutic target in lung fibrosis and metastatic pancreatic cancer
Abstract
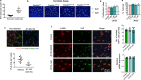
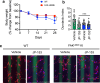
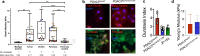
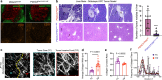
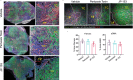
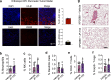
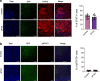
Durotaxis, cell migration along stiffness gradients, is linked to embryonic development, tissue repair and disease. Despite solid in vitro evidence, its role in vivo remains largely speculative. Here we demonstrate that durotaxis actively drives disease progression in vivo in mouse models of lung fibrosis and metastatic pancreatic cancer. In lung fibrosis, durotaxis directs fibroblast recruitment to sites of injury, where they undergo mechano-activation into scar-forming myofibroblasts. In pancreatic cancer, stiffening of the tumour microenvironment induces durotaxis of cancer cells, promoting metastatic dissemination. Mechanistically, durotaxis is mediated by focal adhesion kinase (FAK)-paxillin interaction, a mechanosensory module that links stiffness cues to transcriptional programmes via YAP signalling. To probe this genetically, we generated a FAK-FATL994E knock-in mouse, which disrupts FAK-paxillin binding, blocks durotaxis and attenuates disease severity. Pharmacological inhibition of FAK-paxillin interaction with the small molecule JP-153 mimics these effects. Our findings establish durotaxis as a disease mechanism in vivo and support anti-durotactic therapy as a potential strategy for treating fibrosis and cancer.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.L. is a founder and has a financial interest in both Mediar Therapeutics and Zenon Biotech. The companies are developing treatments for organ fibrosis and cancer related to this work. D.L. has received consulting fees from Merck & Co, Scholar Rock, Ono Pharma, UCB Biopharma, Calico Life Sciences, Johnson & Johnson, Inzen Therapeutics, BioHope and PureTech Health LLC that are not related to this work. D.L. has received research support from Boehringer Ingelheim, Merck & Co, Indalo Therapeutics, Ono Pharma and Unity Biotechnology, which was not used in this work. D.J.T. is a scientific advisor and has a financial interest in Zenon Biotech. D.T. has received consulting fees from ROME Therapeutics, Foundation Medicine, Inc., NanoString Technologies, EMD Millipore Sigma and Pfizer that are not related to this work. D.T. is a founder and has equity in ROME Therapeutics, PanTher Therapeutics and TellBio, Inc., which is not related to this work. D.T. receives research support from ACD-Biotechne, PureTech Health LLC and Ribon Therapeutics, which was not used in this work. D.L.’s and D.T.’s interests were reviewed and are managed by Mass General Brigham in accordance with their conflict-of-interest policies. The other authors declare no competing interests.
Figures









References
-
- Sunyer, R. et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science353, 1157–1161 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

