Antibody-bottlebrush prodrug conjugates for targeted cancer therapy
- PMID: 40925997
- PMCID: PMC12425459
- DOI: 10.1038/s41587-025-02772-z
Antibody-bottlebrush prodrug conjugates for targeted cancer therapy
Abstract
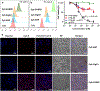
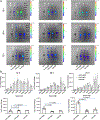
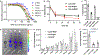
Antibody-drug conjugates (ADCs) are effective targeted therapeutics but are limited in their ability to incorporate less-potent payloads, varied drug mechanisms of action, different drug release mechanisms and tunable drug-to-antibody ratios. Here we introduce a technology to overcome these limitations called 'antibody-bottlebrush prodrug conjugates' (ABCs). An ABC consists of an IgG1 monoclonal antibody covalently conjugated to the terminus of a compact bivalent bottlebrush prodrug that has payloads bound through cleavable linkers and polyethylene glycol branches. This design enables the synthesis of ABCs with tunable average drug-to-antibody ratios up to two orders of magnitude greater than those of traditional ADCs. We demonstrate the functional flexibility and manufacturing efficiency of this technology by synthesizing more than 10 different ABCs targeting either HER2 or MUC1 using drugs with potencies spanning several orders of magnitude as well as imaging agents for ABC visualization and photocatalysts for proximity-based labeling of the ABC interactome. ABCs display high target engagement, high cell uptake and improved efficacy in tumor models compared to conventional HER2-targeted ADCs, suggesting promise for clinical translation.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: J.A.J., H.V.-T.N. and Y.J. are shareholders of Window Therapeutics. D.W.C.M. declares an ownership interest in Dexterity Pharma, which has commercialized materials used in this work. The remaining authors declare no competing interests.
Figures












References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A Cancer statistics, 2022. CA. Cancer J. Clin 72, 7–33 (2022). - PubMed
-
- Hobson AD Chapter One - Antibody drug conjugates beyond cytotoxic payloads. Progress in Medicinal Chemistry. 62, 1–59 (2023). - PubMed
-
- Tarantino P, Ricciuti B, Pradhan SM & Tolaney SM Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat. Rev. Clin. Oncol 20, 558–576 (2023). - PubMed
-
- Dumontet C, Reichert JM, Senter PD, Lambert JM & Beck A Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov 22, 641–661 (2023). - PubMed
Methods-only references
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

