Identification of p38 MAPK inhibition as a neuroprotective strategy for combinatorial SMA therapy
- PMID: 40926051
- PMCID: PMC12514318
- DOI: 10.1038/s44321-025-00303-6
Identification of p38 MAPK inhibition as a neuroprotective strategy for combinatorial SMA therapy
Abstract
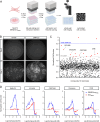
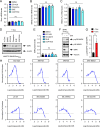
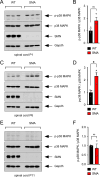
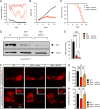
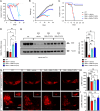
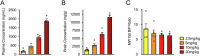
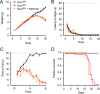
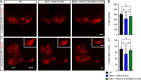
Spinal muscular atrophy (SMA) is a neurodegenerative disease caused by ubiquitous deficiency in the SMN protein. The identification of disease modifiers is key to understanding pathogenic mechanisms and broadening the range of targets for developing SMA therapies that complement SMN upregulation. Here, we report a cell-based screen that identified inhibitors of p38 mitogen-activated protein kinase (p38 MAPK) as suppressors of proliferation defects induced by SMN deficiency in mouse fibroblasts. We further show that SMN deficiency induces p38 MAPK activation and that pharmacological inhibition of this pathway improves motor function in SMA mice through SMN-independent neuroprotective effects. Using a highly optimized p38 MAPK inhibitor (MW150) and combinatorial treatment in SMA mice, we observed synergistic enhancement of the phenotypic benefit induced by either MW150 or an SMN-inducing drug alone. By promoting motor neuron survival, pharmacological inhibition of p38 MAPK synergizes with SMN induction and enables enhanced synaptic rewiring of motor neurons within sensory-motor spinal circuits. These studies identify the p38 MAPK pathway as a therapeutic target and MW150 as a neuroprotective drug for combination therapy in SMA.
Keywords: Combination Therapy; Neuroprotection; Spinal Muscular Atrophy (SMA); Survival Motor Neuron (SMN); p38 Mitogen-Activated Protein Kinase (p38 MAPK).
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures




Update of
-
A chemical screen identifies p38 MAPK inhibition as a candidate neuroprotective strategy for combinatorial SMA therapy.bioRxiv [Preprint]. 2025 Mar 8:2025.03.06.641938. doi: 10.1101/2025.03.06.641938. bioRxiv. 2025. Update in: EMBO Mol Med. 2025 Oct;17(10):2762-2786. doi: 10.1038/s44321-025-00303-6. PMID: 40236193 Free PMC article. Updated. Preprint.
References
-
- Baranello G, Darras BT, Day JW, Deconinck N, Klein A, Masson R, Mercuri E, Rose K, El-Khairi M, Gerber M et al (2021) Risdiplam in type 1 spinal muscular atrophy. N Engl J Med 384:915–923 - PubMed
-
- Bertini E, Dessaud E, Mercuri E, Muntoni F, Kirschner J, Reid C, Lusakowska A, Comi GP, Cuisset JM, Abitbol JL et al (2017) Safety and efficacy of olesoxime in patients with type 2 or non-ambulatory type 3 spinal muscular atrophy: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16:513–522 - PubMed
MeSH terms
Substances
Grants and funding
- R21 NS071092/NS/NINDS NIH HHS/United States
- NS098363/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS102451/NS/NINDS NIH HHS/United States
- NS071092/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS116400/NS/NINDS NIH HHS/United States
- R01 NS114218/NS/NINDS NIH HHS/United States
- R21 NS098363/NS/NINDS NIH HHS/United States
- NS116400/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS114218/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS083831/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R21 NS083831/NS/NINDS NIH HHS/United States
- N/A/Cure SMA (CSMA)
- NS102451/HHS | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources
Medical

