The European Health Data Space in communicable diseases surveillance and monitoring of medicines and vaccines: achievements of a pilot project examining the user journey
- PMID: 40926480
- PMCID: PMC12420905
- DOI: 10.1093/eurpub/ckaf115
The European Health Data Space in communicable diseases surveillance and monitoring of medicines and vaccines: achievements of a pilot project examining the user journey
Abstract
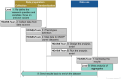
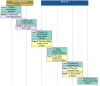
The future European Health Data Space (EHDS), a network for secure cross-border data use, could be beneficial for public health initiatives. The HealthData@EU pilot project evaluated possibilities of secondary data use based on five use cases and established a pilot IT infrastructure. This article reports overarching experiences from two public health use cases and the IT development. Experiences were reported by the Users' Journey steps (data discovery, application, use, and finalization) elaborated in the context of the European Health Data Space (TEHDAS), the first conceptual EHDS project. The European Medicines Agency's use case analysed coagulopathy-related events in COVID-19 patients, the one led by the European Centre for Disease Prevention and Control assessed the feasibility of HealthData@EU to support antimicrobial resistance surveillance. The IT work package developed the infrastructure for information exchange. The use cases indicated that standardized procedures, wherever available, were considered highly beneficial. In the application phase, node-specific requirements slowed down the progress. A distributed approach in combination with the use of Secure Processing Environments (SPE) was successfully conducted. The IT infrastructure was piloted, tested, and published as open source. It supports data discovery and applications in the future. The HealthData@EU pilot project has achieved major steps regarding data discoverability and accessibility, where a need for more standardized procedures was detected in the use cases. Distributed analyses in combination with SPE use may be possible approaches for the data use phase, which require further investigation in TEHDAS2 and other initiatives.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Public Health Association.
Figures
References
-
- European Medicines Agency. Data Analysis and Real World Interrogation Network (DARWIN). https://www.ema.europa.eu/en/about-us/how-we-work/big-data/real-world-ev... (4 November 2024, date last accessed).
-
- Official Journal of the European Union. Regulation (EU) 2022/2370 of the European Parliament and of the Council of 23 November 2022 amending Regulation (EC) No 851/2004 establishing a European centre for disease prevention and control. OJ L 314, 6.12.2022, 1–25. https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=uriserv%3AOJ.L_.2022.... (7 November 2024, date last accessed).
-
- Official Journal of the European Union. Regulation (EU) 2022/2371 of the European Parliament and of the Council of 23 November 2022 on serious cross-border threats to health and repealing Decision No 1082/2013/EU. OJ L 314, 6.12.2022, 26–63. https://eur-lex.europa.eu/legalcontent/EN/TXT/?uri=uriserv%3AOJ.L_.2022.... (4 November 2024, date last accessed).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical