Laser processing in liquids: insights into nanocolloid generation and thin film integration for energy, photonic, and sensing applications
- PMID: 40927487
- PMCID: PMC12415901
- DOI: 10.3762/bjnano.16.104
Laser processing in liquids: insights into nanocolloid generation and thin film integration for energy, photonic, and sensing applications
Abstract
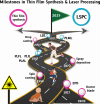
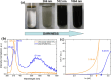
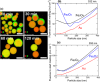
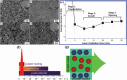
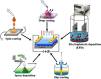
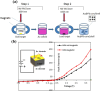
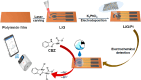
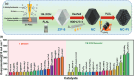
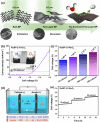
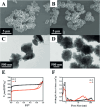
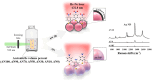
Nanoparticles in their pure colloidal form synthesized by laser-assisted processes such as laser ablation/fragmentation/irradiation/melting in liquids have attained much interest from the scientific community because of their specialties like facile synthesis, ultra-high purity, biocompatibility, colloidal stability in addition to other benefits like tunable size and morphology, crystalline phases, new compounds and alloys, and defect engineering. These nanocolloids are useful for fabricating different devices mainly with applications in optoelectronics, catalysis, sensors, photodetectors, surface-enhanced Raman spectroscopy (SERS) substrates, and solar cells. In this review article, we describe different methods of nanocolloidal synthesis using laser-assisted processes and corresponding thin film fabrication methods, particularly those utilized for device fabrication and characterization. The four sections start with an introduction to the common laser-assisted synthesis for nanocolloids and different methods of thin film fabrication using these nanocolloids followed by devices fabricated and characterized for applications including photovoltaics, photodetectors, catalysis, photocatalysis, electrochemical/photoelectrochemical sensors, hydrogen/oxygen evolution, SERS sensors and other types of devices reported so far. The last section explains the challenges and further scope of these devices from laser-generated nanocolloids.
Keywords: HER/OER/water splitting; laser synthesis of nanomaterials; nanocolloids to thin films; photocatalysis; photovoltaics and photodetection; surface-enhanced Raman spectroscopy (SERS).
Copyright © 2025, Parameswaran Sreekala et al.
Figures














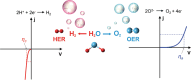

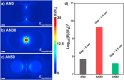

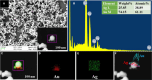


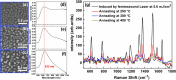

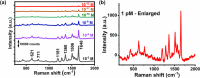

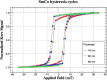

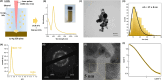
References
-
- von der Linde D, Sokolowski-Tinten K. Appl Surf Sci. 2000;154–155:1–10. doi: 10.1016/s0169-4332(99)00440-7. - DOI
-
- Rangappa S M, Parameswaranpillai J, Siengchin S, editors. Polymer Coatings. Boca Raton, FL, USA: CRC Press; 2020. - DOI
-
- Simakin A V, Voronov V V, Kirichenko N A, Shafeev G A. Appl Phys A: Mater Sci Process. 2004;79:1127–1132. doi: 10.1007/s00339-004-2660-8. - DOI
-
- Zeng H, Du X-W, Singh S C, Kulinich S A, Yang S, He J, Cai W. Adv Funct Mater. 2012;22(7):1333–1353. doi: 10.1002/adfm.201102295. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
