Stakeholder engagement and pharmaceutical pricing regulation: a qualitative inquiry
- PMID: 40927584
- PMCID: PMC12416026
- DOI: 10.1080/20523211.2025.2550370
Stakeholder engagement and pharmaceutical pricing regulation: a qualitative inquiry
Abstract
Background: Medicine affordability is a critical component of a country's redistributive health policies aimed at ensuring equitable access to healthcare. This study aims to investigate key stakeholders' perspectives on pharmaceutical pricing control in Malaysia as the country is moving towards sustainable healthcare.
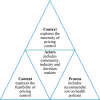
Methods: Semi-structured interviews (n = 16) were conducted with a purposive sampling of key stakeholders, which included practitioners and policymakers engaged in Malaysia's public health policy. Data were analysed using thematic analysis guided by Walt and Gilson's [(1994). Reforming the health sector in developing countries: The central role of policy analysis. Health Policy and Planning, 9(4), 353-370. https://doi.org/10.1093/heapol/9.4.353] Health Policy Triangle framework.
Results: The findings indicate a range of opinions among stakeholders, with most generally favouring the implementation of pharmaceutical pricing regulation. However, concerns have been raised about potential cost transfer, where medication expenses may be shifted to other medical services. Furthermore, there are apprehensions that price controls could adversely affect the profitability of the pharmaceutical industry and impede the development of innovative drugs. Proposed measures include the introduction of price controls and the enhancement of price transparency for specific medications used to address acute and major health issues.
Conclusion: Our study contributes to the current understanding of the formation of public health policies to improve social welfare through stakeholder engagement to ensure that it reflects public needs. Malaysia is a valuable example for developing countries seeking equitable access to manage rising healthcare costs. The study is crucial for understanding country-specific experiences and stakeholders' views on pharmaceutical pricing regulations.
Keywords: Public health; external reference pricing; pharmaceutical pricing policy.
© 2025 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Ashraf, A., & Ong, S. C. (2021). A case for external reference pricing to regulate drug costs in low- and middle-income countries. British Journal of Healthcare Management, 27(10), 1–4. 10.12968/bjhc.2021.0050 - DOI
LinkOut - more resources
Full Text Sources