S-glutathionylation modification of proteins and the association with cellular death (Review)
- PMID: 40927693
- PMCID: PMC12415839
- DOI: 10.3892/mi.2025.263
S-glutathionylation modification of proteins and the association with cellular death (Review)
Abstract
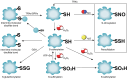
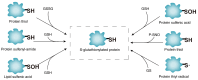
S-glutathionylation (SSG), a redox-sensitive post-translational modification mediated by glutathione, regulates protein structure and function through reversible disulfide bond formation at cysteine residues. Glutaredoxins (GRXs), pivotal antioxidant enzymes, catalyze SSG dynamics to maintain thiol homeostasis. Recent advances in redox proteomics have revealed that SSG dysregulation is intricately linked to neurodegenerative, cardiovascular, pulmonary and malignant diseases. Notably, GRX isoforms (GRX1 and GRX2) play compartment-specific roles in disease pathogenesis: GRX1 modulates hepatic lipid metabolism and pulmonary fibrosis, while GRX2 sustains mitochondrial redox balance and Fe-S cluster assembly. Notably, SSG functions as a 'double-edged sword' in programmed cell death (PCD). While moderate SSG protects against irreversible cysteine oxidation, persistent SSG accumulation due to GRX dysfunction triggers apoptosis, necroptosis and ferroptosis by disrupting redox-sensitive targets, such as caspases, BAX and glutathione peroxidase 4. The present review summarizes, for the first time, at least to the best of our knowledge, the association of SSG with distinct PCD subtypes, and highlights therapeutic strategies targeting GRX activity or site-specific SSG modulation (e.g., pyruvate kinase M2 Cys423/424). Emerging approaches, including GRX mimetics and thiol-targeted drugs, hold promise for precision medicine in redox-related pathologies.
Keywords: S-glutathionylation; glutaredoxins; oxidative stress; programmed cell death; redox signaling.
Copyright: © 2025 Sun et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K. Reactive oxygen species-sources, functions, oxidative damage. Pol Merkur Lekarski. 2020;48:124–127. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
