Unraveling epigenetic drivers of immune evasion in gliomas: mechanisms and therapeutic implications
- PMID: 40927709
- PMCID: PMC12415009
- DOI: 10.3389/fimmu.2025.1633338
Unraveling epigenetic drivers of immune evasion in gliomas: mechanisms and therapeutic implications
Abstract
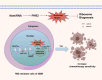
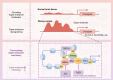
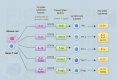
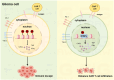
Gliomas are the most common primary malignant tumors of the central nervous system (CNS), and despite progress in molecular diagnostics and targeted therapies, their prognosis remains poor. In recent years, immunotherapy has emerged as a promising treatment modality in cancer therapy. However, the inevitable immune evasion by tumor cells is a key barrier affecting therapeutic efficacy. Epigenetic regulation, such as DNA methylation, histone modification, and non-coding RNA expression, plays a crucial role in the occurrence, development, and immune evasion of gliomas. These modifications can dynamically regulate gene expression, leading to the silencing of tumor-associated antigens, dysregulation of pro-inflammatory cytokines, and dynamic modulation of immune checkpoints (such as PD-L1). This review systematically elucidates the key mechanisms by which epigenetic regulation promotes immune evasion in gliomas and details three interconnected mechanisms: 1) epigenetic silencing of tumor-associated antigens and antigen-presenting machinery; 2) dysregulation of pro-inflammatory cytokine secretion; and 3) dynamic modulation of PD-L1 expression through chromatin remodeling. We emphasize the potential of combining epigenetic therapies with immunotherapies to enhance anti-tumor immune responses and overcome treatment resistance in gliomas. Future research should focus on developing biomarker-driven epigenetic immunotherapies and exploring the complex interplay between epigenetic modifications, glioma cells, and the tumor immune microenvironment to improve patient outcomes.
Keywords: CAR-T cell therapy; epigenetic regulations; gliomas; immune evasion; non-coding RNA.
Copyright © 2025 Wu, Ju, Zhao, Liu, Liu and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

