Drug-Eluting Resorbable Scaffold Versus Balloon Angioplasty for Below-the-Knee Peripheral Artery Disease: 2-Year Results From the LIFE-BTK Trial
- PMID: 40927852
- PMCID: PMC12513053
- DOI: 10.1161/CIRCULATIONAHA.125.075080
Drug-Eluting Resorbable Scaffold Versus Balloon Angioplasty for Below-the-Knee Peripheral Artery Disease: 2-Year Results From the LIFE-BTK Trial
Abstract
Background: Limited treatment options exist for infrapopliteal disease in patients with chronic limb-threatening ischemia (CLTI), a condition associated with a high risk of limb loss. Interventional management of diseased infrapopliteal vessels with percutaneous transluminal angioplasty (PTA) is associated with high rates of restenosis and reintervention. In the LIFE-BTK randomized controlled trial (Pivotal Investigation of Safety and Efficacy of BRS Treatment-Below the Knee), the drug-eluting resorbable scaffold (DRS) demonstrated superior 12-month efficacy compared with PTA in a selected CLTI population with predominantly noncomplex, mildly to moderately calcified lesions. This report presents the 2-year safety and efficacy outcomes of the Esprit BTK DRS system in the LIFE-BTK randomized trial comparing DRS with PTA for treatment of infrapopliteal vessels and CLTI.
Methods: The LIFE-BTK trial was a multicenter, subject-blinded, randomized controlled trial enrolling 261 patients with CLTI who were randomized 2:1 to receive either DRS or PTA. The revised primary efficacy end point was freedom from target limb amputation, target vessel occlusion, clinically driven target lesion revascularization, or binary restenosis. The primary safety end point was freedom from major adverse limb events and perioperative death. Predictors of efficacy and clinically driven target lesion revascularization were analyzed along with subgroup assessments.
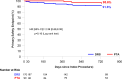
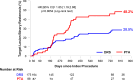
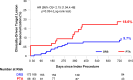
Results: At 2 years, the primary efficacy end point was observed in 68.8% of the DRS group versus 45.4% of the PTA group (P=0.0004). Limb salvage rates were 94.7% for DRS and 97.3% for PTA (P=0.34). Binary restenosis occurred in 28.5% of DRS patients versus 48.2% of PTA patients (P=0.005), and clinically driven target lesion revascularization rates were 9.7% versus 18.6%, respectively (P=0.034). The primary safety end point was observed in 91.6% of the DRS group versus 95.6% of the PTA group (P=0.16). Scaffold treatment was an independent predictor of efficacy (odds ratio, 0.27; P=0.0003) and showed a trend toward reduced risk of clinically driven target lesion revascularization, though this did not reach statistical significance. Other predictors included lesion length, Rutherford-Becker class 5, total occlusion, previous amputation, preintervention stenosis, and number of wounds. Subgroup analyses demonstrated consistent efficacy across various patient populations.
Conclusions: At 2 years, the Esprit BTK DRS demonstrated improved efficacy compared with PTA in maintaining arterial patency, preventing restenosis, and reducing revascularization rates while maintaining a comparable safety profile. These findings support the Esprit BTK scaffold as a promising treatment option for appropriately selected patients with infrapopliteal artery disease and CLTI.
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT04227899.
Keywords: DRS; balloon angioplasty; binary restenosis; chronic limb-threatening ischemia; drug-eluting resorbable scaffold; infrapopliteal artery disease; vascular patency.
Conflict of interest statement
Dr DeRubertis is a consultant for Abbott Vascular, Boston Scientific, Bard Peripheral, Cagent Vascular, Concept Medical, and Medtronic. Dr Varcoe is a consultant for Medtronic, Abbott Vascular, BD, Intervene, Surmodics, Philips, Nectero, Endospan, Boston Scientific, Vesteck, W.L. Gore, R3 Vascular, Cook Medical, and Concept Medical. He holds equity in Provision Medical, Inc. and Vesteck. Dr Krishnan is a consultant for Medtronic and Abbott. Dr Bonaca is the executive director of the Colorado Prevention Center, a nonprofit academic research organization affiliated with the University of Colorado, which receives or has received research grant or consulting funding from Abbott Laboratories, Agios Pharmaceuticals, Alexion Pharma, Alnylam Pharmaceuticals, Amgen, Angionetics, Anthos Therapeutics, Array BioPharma, AstraZeneca and affiliates, Atentiv, Audentes Therapeutics, Bayer and affiliates, Bristol Myers Squibb, Cambrian Biopharma, Cardiol Therapeutics, CellResearch, Cleerly, Cook Regentec, CSL Behring, Eidos Therapeutics, EP Trading, Epizon Pharma, Esperion Therapeutics, Everly Well, Exicon Consulting, Faraday Pharmaceuticals, Foresee Pharmaceuticals, Fortress Biotech, HDL Therapeutics, HeartFlow, Hummingbird Bioscience, Insmed, Ionis Pharmaceuticals, Janssen and Affiliates, Kowa Research Institute, Lexicon Pharmaceuticals, Medimmune, Merck and affiliates, Nectero Medical, Novartis Pharmaceuticals, Novo Nordisk, Osiris Therapeutics, Pfizer, PhaseBio Pharmaceuticals, Prairie Education and Research Cooperative, Prothena Biosciences, Regeneron Pharmaceuticals, Regio Biosciences, Sanofi-Aventis Group, Silence Therapeutics, Smith & Nephew, Stealth BioTherapeutics, VarmX, and Virta Health Corporation. Dr O’Connor serves as a speaker and faculty for Shockwave Medical. He is a principal investigator for a study funded by Boston Scientific and has received a research grant from Abbott Medical. Dr Pin is on the advisory board for Abbott and Boston Scientific. Dr Metzger is a consultant for Abbott Vascular, Boston Scientific, Endologix, Penumbra, and Shockwave Medical. Dr Holden is a consultant for Boston Scientific, Medtronic, Philips, and W.L. Gore & Associates, Inc. and is a clinical investigator for studies funded by Bard-BD, Boston Scientific, Cagent Medical, Cook Medical, Efemoral, Endologix, Endospan, Gore Medical, Intact Vascular, Medtronic, Nectero, Philips, Reflow Medical, Shape Memory, Shockwave Medical, and Terumo. Dr Iida is on the advisory board for Abbott, Boston Scientific, and Cordis. He is a consultant for Abbott, Boston Scientifics, and Otsuka Medical and has received honoraria from Boston Scientific Japan, Becton Dickinson, Cordis, W.L. Gore, Medtronic Japan, and Terumo Co., Ltd. Dr Armstrong is a consultant to Abbott Vascular, BD, Cordis, Gore, Shockwave Medical, REVA medical, and AngioDynamics. Dr Kum is a consultant for Abbott Vascular. Dr Kolluri is a consultant/advisor for Abbott, Auxetics, Boston Scientific, Daiichi Sankyo, Koya Medical, Medtronic, Penumbra, Philips, and Surmodics. He is a board trustee of the VIVA Foundation and the Intersocietal Accreditation Council for Vascular Testing and President of Syntropic Corelab. Dr Bajakian is on the advisory board for Abbott Vascular and Boston Scientific. Dr Garcia is a consultant for MDTC, Boston Scientific, Phillips, and Abbott. He holds equity interests in R3, Cagent, Tissue Gen, Transit Medical, Primacea, and Sintervention. He is the owner and founder of Innovation Vascular Partners, LLC. Dr Shishehbor is a consultant and global advisor for Medtronic, Abbott Vascular, Boston Scientific, Cordis, Philips, ANT, Inquis, and Inari (Stryker). Dr Parikh is a consultant for Abiomed, Terumo, Abiomed, Penumbra, and Canon. He holds equity interests in Encompass Vascular, Adv NanoTherapies, and eFemoral. He is on the advisory board for Abbott, Medtronic, Boston Scientific, Cordis, and Philips and has received institutional research support from Abbott, Acotec, Concept Medical, Shockwave Medical, Boston Scientific, Reflow Medical, Fastwave, Veryan Medical, Philips, Cagent Vascular, Medinol, and AVS. Dr Yu, Dr Ruster, Dr Martinsen, and Dr Igyarto are employed by and own stock in Abbott Vascular.
Figures

References
-
- Moschiar Almeida B, Evans R, Kayssi A. Fundamentals of wound care for amputation prevention. Semin Vasc Surg. 2025;38:54–63. doi: 10.1053/j.semvascsurg.2025.01.001 - PubMed
-
- Farber A. Chronic limb-threatening ischemia. N Engl J Med. 2018;379:171–180. doi: 10.1056/NEJMcp1709326 - PubMed
-
- Bradbury AW, Moakes CA, Popplewell M, Meecham L, Bate GR, Kelly L, Chetter I, Diamantopoulos A, Ganeshan A, Hall J, et al. ; BASIL-2 Investigators. A vein bypass first versus a best endovascular treatment first revascularisation strategy for patients with chronic limb threatening ischaemia who required an infra-popliteal, with or without an additional more proximal infra-inguinal revascularisation procedure to restore limb perfusion (BASIL-2): an open-label, randomised, multicentre, phase 3 trial. Lancet. 2023;401:1798–1809. doi: 10.1016/S0140-6736(23)00462-2 - PubMed
-
- Cha J-J, Kim J-Y, Kim H, Ko Y-G, Choi D, Lee J-H, Yoon C-H, Chae I-H, Yu CW, Lee SW, et al. ; K-VIS (Korean Vascular Intervention Society) investigatorsvestigators on behalf of the K-V (Korean VIS. Long-term clinical outcomes and prognostic factors after endovascular treatment in patients with chronic limb threatening ischemia. Korean Circ J. 2022;52:429–440. doi: 10.4070/kcj.2021.0342 - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

