Inner Limiting Membrane Peel Extends In Vivo Calcium Imaging of Retinal Ganglion Cell Activity Beyond the Fovea in Non-Human Primate
- PMID: 40928310
- PMCID: PMC12429706
- DOI: 10.1167/iovs.66.12.25
Inner Limiting Membrane Peel Extends In Vivo Calcium Imaging of Retinal Ganglion Cell Activity Beyond the Fovea in Non-Human Primate
Abstract
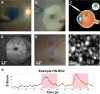
Purpose: Adaptive optics scanning light ophthalmoscopy (AOSLO) paired with intravitreal injection of a viral vector coding for the calcium indicator GCaMP has enabled visualization of neuronal activity in retinal ganglion cells (RGCs) at single cell resolution in the living eye. However, the inner limiting membrane (ILM) restricts viral transduction to the fovea in humans and non-human primates, hindering both therapeutic intervention and physiological study of the retina. To address this issue, we explored peeling the ILM before intravitreal injection to expand calcium imaging beyond the fovea in the living primate eye.
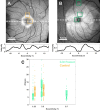
Methods: Five eyes from three Macaca fascicularis (aged 3-10 years; 2 males, 1 female) that were immune suppressed with cyclosporine, underwent vitrectomy and ILM peel centered on the fovea prior to intravitreal delivery of 7m8:SNCG:GCaMP8. RGC responses to visual flicker were evaluated using AOSLO calcium imaging 1 to 6 months after intravitreal injection.
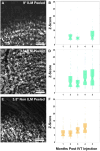
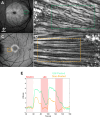
Results: Calcium activity was observed in RGCs throughout the ILM peeled area in all eyes, representing a mean eight-fold increase in accessible recording area relative to a representative control eye. RGC responses in the ILM peeled and control eyes were comparable and showed no significant decrease over the 6 month period after the procedure. In addition, we demonstrated that activity can be recorded directly from the retinal nerve fiber layer.
Conclusions: Peeling the ILM is a viable strategy to expand viral access to RGCs for gene therapy, and when paired with GCaMP imaging has the potential to advance visual neuroscience, preclinical evaluation of retinal function, detection of vision loss, and assessment of therapeutic interventions.
Conflict of interest statement
Disclosure:
Figures


Update of
-
Inner limiting Membrane Peel Extends In vivo Calcium Imaging of Retinal Ganglion Cell Activity Beyond the Fovea in Non-Human Primate.bioRxiv [Preprint]. 2025 Mar 7:2024.06.02.597041. doi: 10.1101/2024.06.02.597041. bioRxiv. 2025. Update in: Invest Ophthalmol Vis Sci. 2025 Sep 2;66(12):25. doi: 10.1167/iovs.66.12.25. PMID: 38854047 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

