Acute REM sleep deprivation alleviated depression-like behavior mediated by inhibiting VIP neurons in the mPFC
- PMID: 40929273
- PMCID: PMC12422183
- DOI: 10.1126/sciadv.adx2666
Acute REM sleep deprivation alleviated depression-like behavior mediated by inhibiting VIP neurons in the mPFC
Abstract
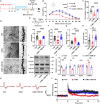
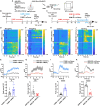
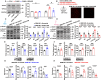
Acute sleep deprivation (SD) rapidly alleviates depression, addressing a critical gap in mood disorder treatment. Rapid eye movement SD (REM SD) modulates the excitability of vasoactive intestinal peptide (VIP) neurons, influencing the synaptic plasticity of pyramidal neurons. However, the precise mechanism remains undefined. To investigate this, we used a modified multiple platform method (MMPM) to induce 12 hours of REM SD, specifically targeting VIP neurons in the medial prefrontal cortex (mPFC). Our results show that REM SD mitigated depression by suppressing VIP neurons activity, which directly increased the excitability of pyramidal neurons and, consequently, promoted synaptic plasticity recovery. In addition, the knockdown of VPAC2 on mPFC pyramidal neurons revealed that VPAC2-mediated AC/cAMP/PKA signaling pathway in these neurons is essential for REM SD to mitigate depression-like behavior. These findings suggest that VIP neurons directly regulate pyramidal neurons and are crucial in alleviating depression by REM SD.
Figures



References
-
- Li X., Zhang H., Wu K., Fan B., Guo L., Liao Y., McIntyre R. S., Wang W., Liu Y., Shi J., Chen Y., Shen M., Wang H., Li L., Han X., Lu C., Impact of painful physical symptoms on first-episode major depressive disorder in adults with subthreshold depressive symptoms: A prospective cohort study. Gen. Hosp. Psychiatry 86, 1–9 (2024). - PubMed
-
- Van Loh B. M., Yaw A. M., Breuer J. A., Jackson B., Nguyen D., Jang K., Ramos F., Ho E. V., Cui L. J., Gillette D. L. M., Sempere L. F., Gorman M. R., Tonsfeldt K. J., Mellon P. L., Hoffmann H. M., The transcription factor VAX1 in VIP neurons of the suprachiasmatic nucleus impacts circadian rhythm generation, depressive-like behavior, and the reproductive axis in a sex-specific manner in mice. Front. Endocrinol. 14, 1269672 (2023). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

