Concomitant use of anti-leishmanial therapy and antibacterial prophylaxis reduces plasma LPS levels and improves several aspects of experimental Leishmania infantum infection in golden hamsters
- PMID: 40929455
- PMCID: PMC12418786
- DOI: 10.1590/0074-02760240266
Concomitant use of anti-leishmanial therapy and antibacterial prophylaxis reduces plasma LPS levels and improves several aspects of experimental Leishmania infantum infection in golden hamsters
Abstract
Background: Parasite antigens and plasma lipopolysaccharide (LPS) levels from luminal origin in visceral leishmaniasis (VL) patients are correlated with cellular activation and low CD4+T cell counts.
Objectives: Our aim was to verify whether Leishmania infantum infection damages the intestinal barrier and whether combination antimonial/antibiotic contributes to the reduction of LPS levels and immune activation.
Methods: Golden hamsters were grouped in: G1-uninfected; G2-infected with L. infantum; and G3/G4 and G5-infected, treated with antimonial, antibiotic or both drugs, respectively. The treatment initiated 45 days post infection (dpi), daily by 10 days.
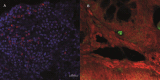
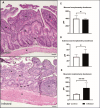
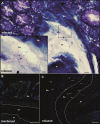
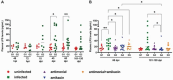
Findings: G2, G3, and G4 animals showed a significant increase in spleen weight compared to G1. An elevated parasite load was observed in G2, unlike the G3, G4, and especially, G5, whose decrease was significant at 120 dpi. Intestinal mucosal alterations and elevated LPS levels were observed in G2 group. However, G3, G4 and G5 animals showed lower LPS levels than G2. Moreover, G4 and G5 presented higher CD4+T-cell percentages and lower activation levels than G2 and G3, either at 60 or 101-120 dpi.
Main conclusions: Our results showed evidence of bacterial translocation in experimental VL and that the concomitant use of antimonial and antibiotic may reduce LPS levels, along with an improvement of the immunosuppression and reduction of lymphocyte activation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- MS Situação epidemiológica da leishmaniose visceral. Ministério da Saúde. 2022 https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/l/leishmaniose-vi...
-
- Colmenares M, Kar S, Goldsmith-Pestana K, McMahon-Pratt D. Mechanisms of pathogenesis: differences amongst Leishmania species. Trans R Soc Trop Med Hyg. 2002 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

