Cytoskeletal disruption-induced calcium dysregulation drives cell death in anti-IgLON5 disease
- PMID: 40930030
- PMCID: PMC12454665
- DOI: 10.1016/j.redox.2025.103854
Cytoskeletal disruption-induced calcium dysregulation drives cell death in anti-IgLON5 disease
Abstract
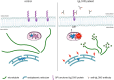
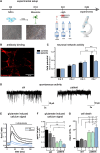
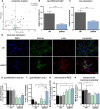
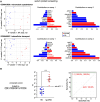
Anti-IgLON5 disease is an autoimmune encephalitis with more chronic presentation including memory decline, sleep disorder, bulbar symptoms and movement disorder. Post-mortem brains of patients with anti-IgLON5 disease show neurodegeneration with tau deposition sparking interest in this 'acquired tauopathy' as a disease model for neurodegeneration, yet mechanisms of neurodegeneration remain unknown. Using a reductionist human iPSC-derived neuron-antibody model, we applied proteomics approach, electrophysiology and live cell imaging. iNeurons treated with anti-IgLON5 IgG presented with cytoskeletal disruption along with tau depositions, which correlated with endophenotypes. Accompanying calcium dysregulation was driven by impaired ER refill and mitochondrial dysfunction leading to cell death. Analogous cytoskeletal disruption is also reflected in the serum of treatment naïve patients using OLink proteomics. These findings provide insight into anti-IgLON5 disease pathology and pinpoint downstream signalling events of direct antibody-neuron interactions, which involve novel targets such as cytoskeletal disruption along with calcium dysregulation.
Keywords: AIE; Calcium dysregulation; Cytoskeletal disruption; Disease phenotype; IgLON5.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest Nothing to report.
Figures

References
-
- Ranaivoson F.M., Turk L.S., Ozgul S., Kakehi S., Daake S. von, Lopez N., Trobiani L., Jaco A. de, Denissova N., Demeler B., Özkan E., Montelione G.T., Comoletti D. A proteomic screen of neuronal cell-surface molecules reveals IgLONs as structurally conserved interaction modules at the synapse. Structure (London, England : 1993) 2019;27(6):893–906.e9. doi: 10.1016/j.str.2019.03.004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

